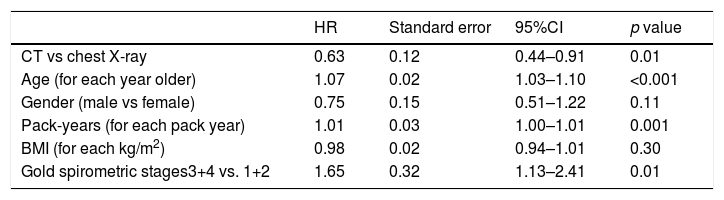
Lung Cancer (LC) screening with low dose chest computed tomography (LDCT) in smokers reduces LC mortality. Patients with Obstructive Lung Disease (OLD) are at high risk for LC. The potential effect of LC screening in this population is unknown.
ObjectiveTo determine if screening with LDCT reduces LC mortality in smokers with spirometrically defined OLD.
MethodsThe National Lung Screening Trial-American College of Radiology Imaging Network (NLST-ACRIN) study included 13,831 subjects (55–74 years of age with ≥30 pack-year history of smoking) that had a baseline spirometry. Randomly assigned to LDCT or Chest X-ray, all had 3 annual rounds of screening. LC mortality was compared between the LDCT and chest X-ray arms during the 1st year and at 6 years of follow up. Landmark analysis explored LC mortality differences between arms after the first year.
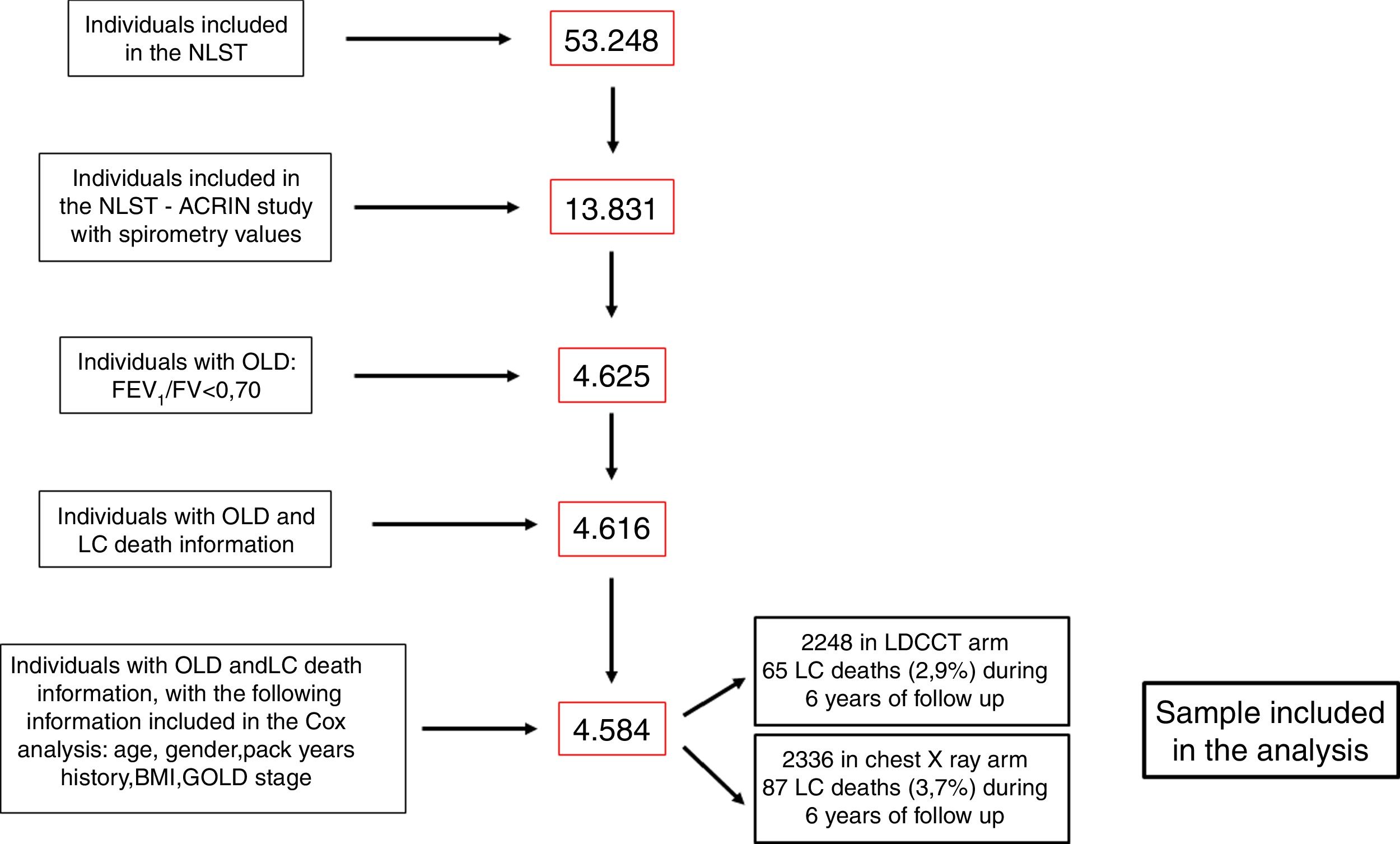
ResultsFrom the 4584 subjects with OLD (FEV1/FVC <0.7), 152 (3.3%) died from LC. Multivariable analysis showed that screening trended to decrease LC mortality at 6 years (HR, 95%CI: 0.75, 0.55–1.04, p=0.09). During the 1st year no differences were found between arms (p=0.65). However, after this year, LDCT significantly decreased LC mortality (HR, 95%CI: 0.63, 0.44–0.91, p=0.01). The number needed to screen to avoid one LC death in these subjects was 108 while in those without OLD was 218.
ConclusionsLC screening with LDCT in smokers with spirometrically diagnosed OLD, showed a trend to reduce lung cancer mortality but a study with a larger number of patients and with a more robust design would be needed to confirm these findings.
El cribado de cáncer de pulmón (CP) utilizando la tomografía computarizada de baja dosis (LDCT, por sus siglas en inglés) de tórax en fumadores reduce la mortalidad por CP. Los pacientes con enfermedad pulmonar obstructiva (EPO) tienen un riesgo alto de presentar CP. Se desconoce el posible efecto del cribado de CP en esta población.
ObjetivoDeterminar si el cribado con LDCT reduce la mortalidad por CP en los fumadores con EPO diagnosticada mediante espirometría.
MétodosEl estudio de cribado de pulmón National Lung Screening Trial-American College of Radiology Imaging Network (NLST-ACRIN) incluyó a 13.831 sujetos (de entre 55-74 años de edad, con historia de tabaquismo de ≥30 paquetes-años) a los que se les había realizado una espirometría basal. Se los asignó aleatoriamente a LDCT o radiografía de tórax y todos pasaron por 3 rondas anuales de cribado. La mortalidad por CP se comparó entre los brazos del LDCT y radiografía de tórax durante el primer año y a los 6 años de seguimiento. El análisis de supervivencia con punto temporal de referencia (landmark analysis) estudió las diferencias en la mortalidad por CP entre los brazos después del primer año.
ResultadosDe los 4.584 sujetos con EPO (FEV1/FVC < 0,7), 152 (3,3%) murieron por CP. El análisis multivariante mostró que el cribado tendía a disminuir la mortalidad por CP a los 6 años (HR: 0,75, IC del 95%; 0,55-1,04, p = 0,09). Durante el primer año no se encontraron diferencias entre los brazos (p = 0,65). Sin embargo, después del año, la LDCT disminuyó significativamente la mortalidad por CP (HR: 0,63, IC del 95%: 0,44-0,91, p = 0,01). El número necesario de cribados para evitar una muerte por CP en estos sujetos fue 108, mientras que en aquellos sin EPO fue 218.
ConclusionesEl cribado de CP con LDCT en fumadores con EPO diagnosticada mediante espirometría mostró una tendencia a reducir la mortalidad por cáncer de pulmón, pero sería necesario un estudio con un mayor número de pacientes y con un diseño más robusto para confirmar estos hallazgos.
The National Lung Screening Trial (NLST)1 and the NEderlands Leuvens Longkanker Screenings ONderzoek (NELSON),2 demonstrated that screening for lung cancer with a low dose chest Computed Tomography (LDCT) significantly decreases lung cancer (LC) mortality. Several studies have also demonstrated that smokers with spirometrically defined Obstructive Lung Disease (OLD), a FEV1/FVC ratio<0.70, have a 2–3 times higher risk of developing LC than those without OLD.3–5
Previous studies suggest that performing LC screening in this high-risk population could decrease the proportion of over diagnosis, cause a positive stage shift3 and may potentially decrease LC mortality.6 To our knowledge, no study has yet explored this hypothesis.
The American College of Radiology Image Network (ACRIN) branch of the NLST study, included 18,831 participants from 23 centers, had a baseline spirometry performed3 and underwent 3 rounds of screening after randomly being assigned to either LDCTs or chest roentgenogram, with subsequent follow-up for 6 years. We therefore studied this cohort to explore the hypothesis that screening patients with OLD with LDCT results in a reduction in mortality from lung cancer as compared to screening with chest X-ray.
MethodsOriginal data collection for ACRIN 6654 (NLST) was supported by NCI Cancer Imaging Program grants. The methodology of this study has been previously described.1 In the ACRIN cohort of the NLST, participants from 23 centers agreed to take part in the study, which included baseline prebronchodilator spirometry. The study was approved by the local Institutional Review Board (IRB) at each screening center. There was no specific criterion in the selection of centers or patients. Unfortunately only pre-bronchodilator spirometry measurements were performed without pletismography or diffusing capacity. A pre-bronchodilator spirometry was done at baseline screening.7 Age, gender, smoking history, body mass index (BMI) and spirometry values were registered: Forced Expiratory Volume in the 1st second (FEV1), Forced Vital Capacity (FVC) and FEV1/FVC.6 OLD is defined by an FEV1/FVC ratio<0.70 as per the Global Initiative for Chronic Obstructive Lung Disease (GOLD) definition.8 All LDCTs were interpreted by trained radiologists.
All participants completed a questionnaire regarding their vital status semiannually. Name and Social Security numbers of those lost to follow-up were submitted to the National Death Index to ascertain probable vital status. As previously described,3 death certificates were obtained for participants who were known to have died. An end-point verification team determined whether the cause of death was lung cancer. Although a distinction was made between a death caused by lung cancer and a death that resulted from the diagnostic evaluation for or treatment of lung cancer, the deaths from the latter causes were counted as lung-cancer deaths in the primary end-point analysis.
Statistical analysisContinuous data with a normal distribution were expressed using mean and standard deviation (SD). Categorical data were described using counts and relative frequencies. Differences between groups were explored with the Student t test or the Chi square test, accordingly.
Overall survival over follow-up time was calculated using Kaplan–Meier method, either for full follow-up or after one year of follow-up with landmark analysis,9 removing all patients with death by lung cancer before the end of the first year or with total follow-up time smaller than 365 days. Univariable and multivariable Cox regression models were used to assess the unadjusted and adjusted effects of the study arm on survival. The selection of the variables included in the multivariate analysis was based on our previous works that showed that these factors were independently related with lung cancer diagnosis and mortality in COPD patients.10,11
Significance was established at a two-tailed p-value<0.05. All statistical analyses were done with SPSS version 20.0 Inc. (IBM, Chicago, IL, USA) and Stata v12.1 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
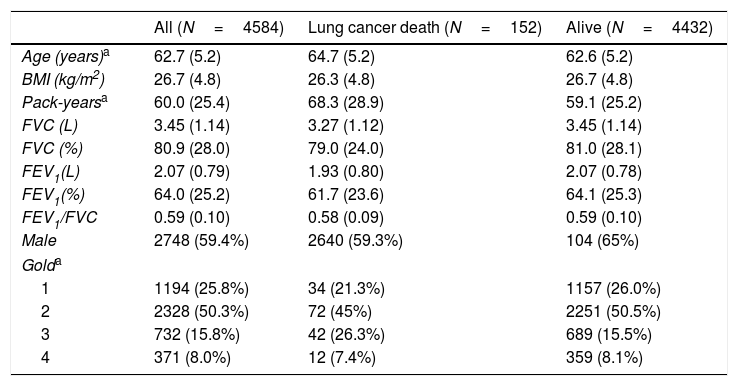
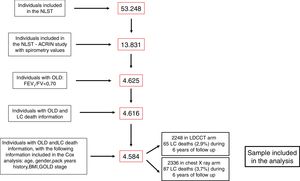
ResultsFig. 1 shows the flowchart of the NLST-ACRIN participants included in the present study. Four thousand five hundred and eighty-four participants with OLD were finally included in the analysis: 2248 underwent screening with a LDCT and 2336 with a chest X-ray. During the follow-up time (6 years), 65 participants (2.9%) died of LC in the LDCT arm and 87 (3.7%) died in the chest X-ray arm. The characteristics of participants who died from LC and who survived until the end of follow-up are shown in Table 1. Participants that died from LC were older, had smoked more pack-years, and had more severe spirometric GOLD stages.
Participant's characteristics.
| All (N=4584) | Lung cancer death (N=152) | Alive (N=4432) | |
|---|---|---|---|
| Age (years)a | 62.7 (5.2) | 64.7 (5.2) | 62.6 (5.2) |
| BMI (kg/m2) | 26.7 (4.8) | 26.3 (4.8) | 26.7 (4.8) |
| Pack-yearsa | 60.0 (25.4) | 68.3 (28.9) | 59.1 (25.2) |
| FVC (L) | 3.45 (1.14) | 3.27 (1.12) | 3.45 (1.14) |
| FVC (%) | 80.9 (28.0) | 79.0 (24.0) | 81.0 (28.1) |
| FEV1(L) | 2.07 (0.79) | 1.93 (0.80) | 2.07 (0.78) |
| FEV1(%) | 64.0 (25.2) | 61.7 (23.6) | 64.1 (25.3) |
| FEV1/FVC | 0.59 (0.10) | 0.58 (0.09) | 0.59 (0.10) |
| Male | 2748 (59.4%) | 2640 (59.3%) | 104 (65%) |
| Golda | |||
| 1 | 1194 (25.8%) | 34 (21.3%) | 1157 (26.0%) |
| 2 | 2328 (50.3%) | 72 (45%) | 2251 (50.5%) |
| 3 | 732 (15.8%) | 42 (26.3%) | 689 (15.5%) |
| 4 | 371 (8.0%) | 12 (7.4%) | 359 (8.1%) |
Mean±standard deviations or n (%).
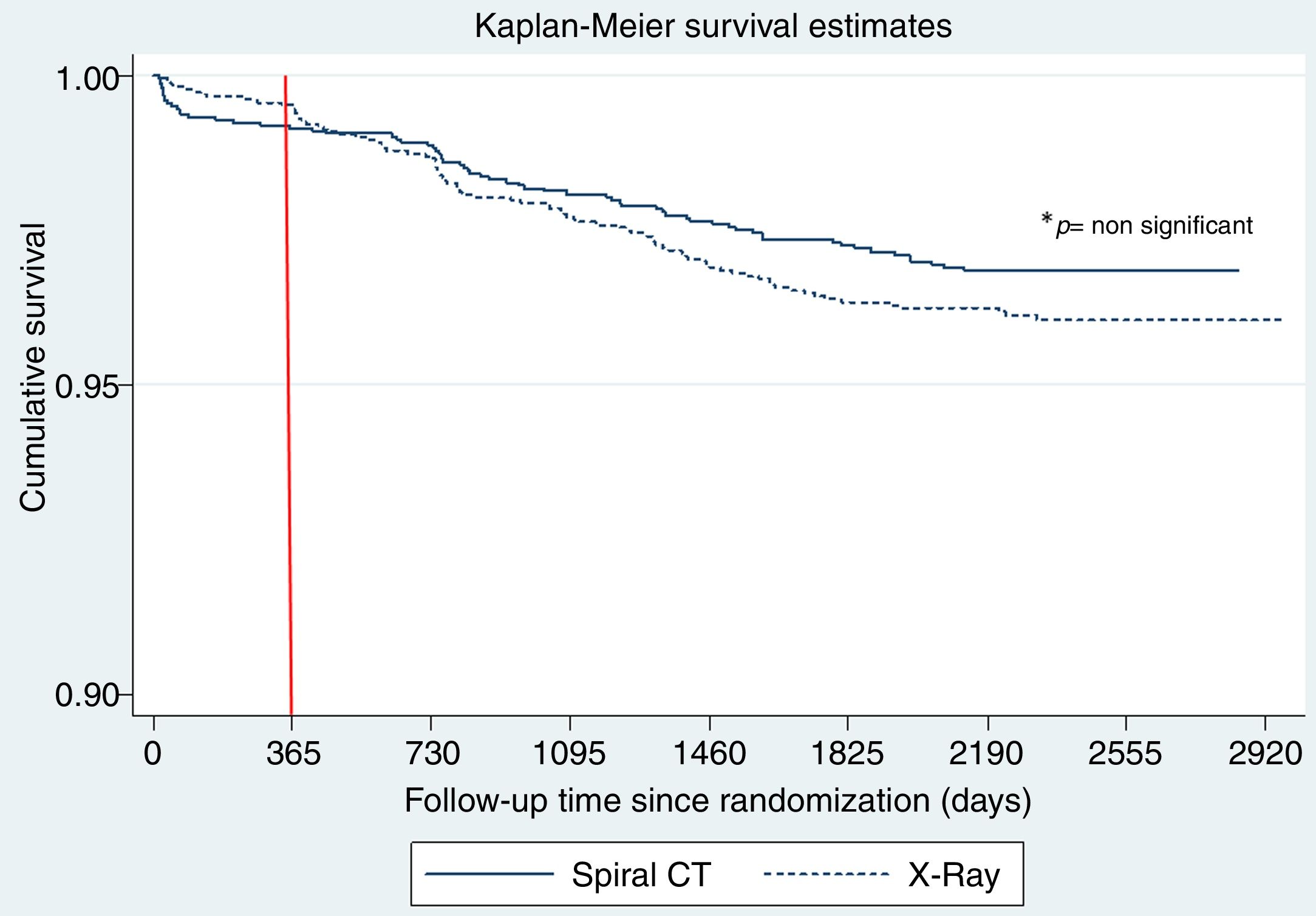
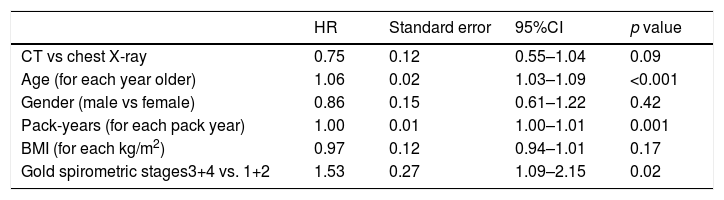
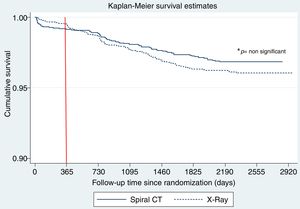
Fig. 2 shows the Kaplan Meier LC survival curves for each interventional arm (LDCT vs chest X-ray), and Table 2 shows the multivariable Cox regression analysis for LC survival comparing both arms and adjusting for other important LC risk factors such as age, gender, BMI, pack years’ history and spirometric GOLD stages. Although a trend for a positive effect of screening with LDCT on LC mortality can be seen in the Cox analysis (a decrease of 25%), it did not reach statistical significance. A complementary analysis to explore the statistical power to determine the impact of LDCT screening on LC mortality in the present sample was performed. This analysis indicated that in order to detect a 25% decrease in LC mortality, we would have needed at least 400 LC deaths to have a 80% statistical power, suggesting that the present study is underpowered (only 152 observed events) to demonstrate that effect.
Multivariable Cox regression analysis of LC survival.
| HR | Standard error | 95%CI | p value | |
|---|---|---|---|---|
| CT vs chest X-ray | 0.75 | 0.12 | 0.55–1.04 | 0.09 |
| Age (for each year older) | 1.06 | 0.02 | 1.03–1.09 | <0.001 |
| Gender (male vs female) | 0.86 | 0.15 | 0.61–1.22 | 0.42 |
| Pack-years (for each pack year) | 1.00 | 0.01 | 1.00–1.01 | 0.001 |
| BMI (for each kg/m2) | 0.97 | 0.12 | 0.94–1.01 | 0.17 |
| Gold spirometric stages3+4 vs. 1+2 | 1.53 | 0.27 | 1.09–2.15 | 0.02 |
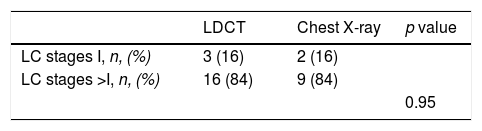
The behavior of the cumulative mortality curves shown in Fig. 2 establishes two clear patterns: during the 1st year of follow-up, both arms seem to have similar curves. However, during the subsequent years of follow-up after the 2nd round of screening, a potentially beneficial effect of LDCT over chest X-ray on LC mortality is observed. Table 3 shows the number of LC deaths that occurred during the first year of follow-up and the stage of the cancer at the time of diagnosis. The distribution and number of lung cancers in individuals who died was similar in both arms (p=0.95).
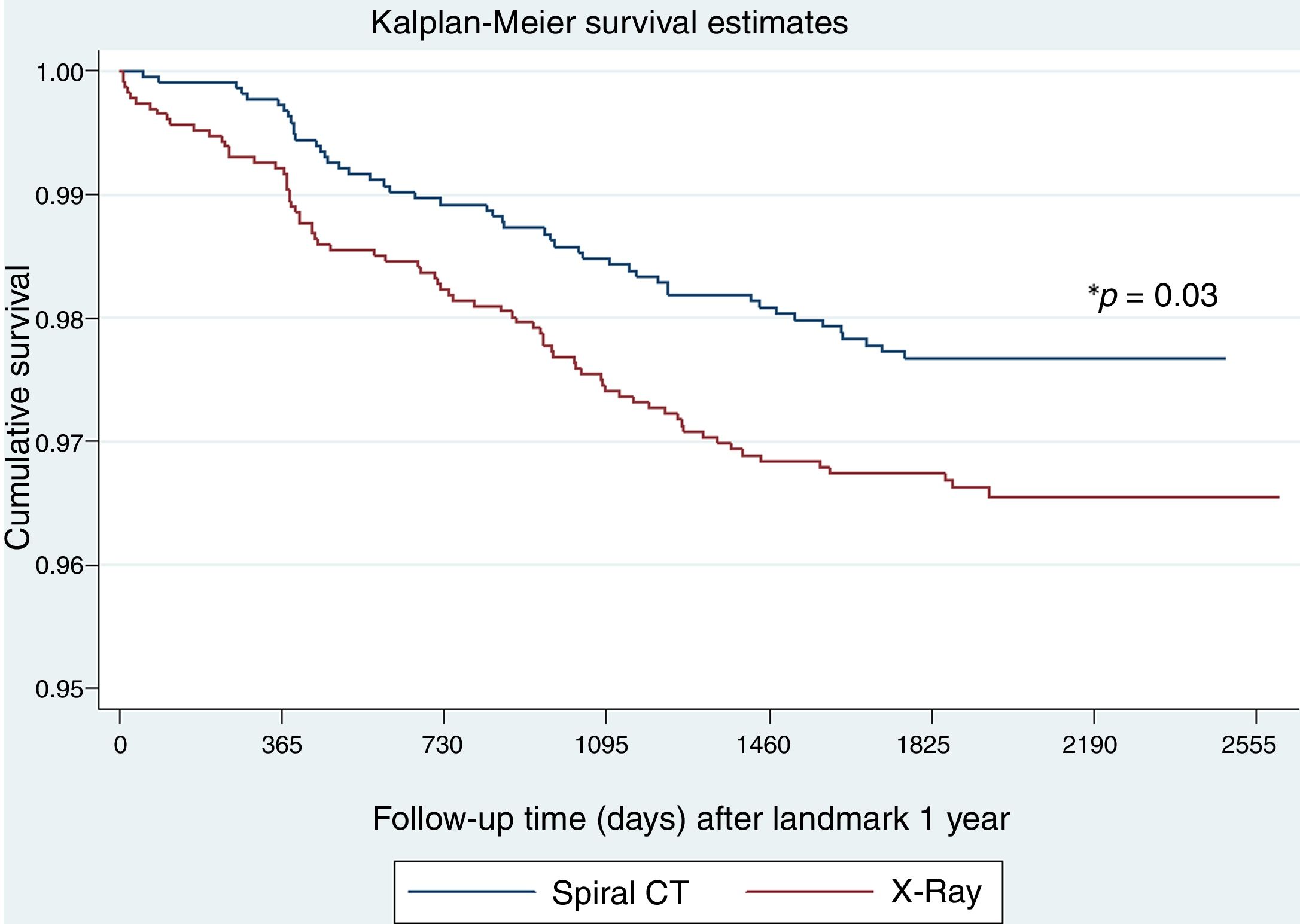
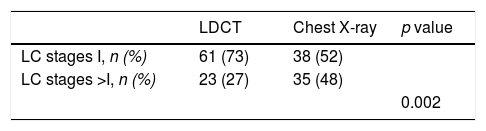
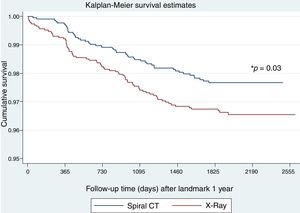
To explore whether LC screening with LDCT in patients with OLD is effective once prevalent cancers detected in the first round of screening are excluded, we analyzed the effect on mortality starting from the second round of screening. There were fewer LC deaths in the LDCT arm as compared to the chest X-ray arm (48 vs 75, respectively, p=0.03) (Fig. 3). Table 4 shows the oncologic stage at the time of diagnosis of patients diagnosed with LC after the first year who were alive at the end of follow-up. A significantly higher proportion of patients were in stage I in the LDCT arm than in the chest X-ray arm (73 vs 52, p=0.002).
Table 5 shows the multivariable Cox regression analysis of LC survival for the landmark analysis after the first year of follow-up. This analysis shows a significant benefit of performing a LDCT vs chest X-ray with a 37% reduction in LC deaths after 5 years of follow-up. The number of participants needed to screen in order to avoid one LC death in this high-risk population was 108 individuals, which compares to 218 in the cohort of participants without spirometrically defined OLD (see Appendix).
Multivariable Cox regression analysis of LC survival for landmark analysis after 1st year.
| HR | Standard error | 95%CI | p value | |
|---|---|---|---|---|
| CT vs chest X-ray | 0.63 | 0.12 | 0.44–0.91 | 0.01 |
| Age (for each year older) | 1.07 | 0.02 | 1.03–1.10 | <0.001 |
| Gender (male vs female) | 0.75 | 0.15 | 0.51–1.22 | 0.11 |
| Pack-years (for each pack year) | 1.01 | 0.03 | 1.00–1.01 | 0.001 |
| BMI (for each kg/m2) | 0.98 | 0.02 | 0.94–1.01 | 0.30 |
| Gold spirometric stages3+4 vs. 1+2 | 1.65 | 0.32 | 1.13–2.41 | 0.01 |
The main finding of this study is that screening patients with OLD for lung cancer using LDCT results in a significant reduction in lung cancer mortality as compared to screening with chest x-ray. The reduction in mortality is significant after excluding subjects with LC diagnosed during the baseline round of screening. Furthermore, the number of screenings needed to be done to save one death from LC (n=108) is much lower than in those without OLD (n=218).
The NLST1 showed that lung cancer screening using LDCT for as few as 3 rounds of screening reduces LC-mortality by at least 20%. Previous studies have also shown that, in comparison to smokers without OLD, those with OLD (defined by an FEV1/FVC<0.70), have a 2–3 fold increased the risk of having lung cancer.3–5 Although no study has yet addressed the effect of LC screening on mortality of individuals with OLD, a review of the NLST did reveal that they benefit from a positive LC stage shift and are less likely to be over diagnosed (i.e. lower prevalence of adenocarcinoma in situ).3
The present retrospective analysis of the prospectively recruited NLST-ACRIN database demonstrated that screening spirometrically confirmed OLD smokers for LC using LDCT, showed a trend to reduce lung cancer mortality in these individuals. Our initial analysis found that although the hazard ratio showed a 25% decrease in mortality (HR 0.75 95%CI: 0.55–1.04, p=0.09) the difference did not reach statistical significance (p=0.09) probably because of the sample size (mainly number of LC deaths needed to demonstrate a statistical difference). However, a closer look at the LC survival curves from the Kaplan–Meier analysis shown in Fig. 2 provided a better perspective of the survival profile of each interventional arm.
During the first year after the baseline screening, subjects in both arms had a similar LC mortality (p=0.95), a behavior that is similar to that reported in the original published NLST work (Fig. 1 panel B),1 where it can be seen that during the first year of follow-up, the number of LC deaths in each arm was identical in the entire screening population.
The novelty in the current work comes from the landmark analysis of LC survival in subjects with OLD after the first year of follow-up or in other words after the 2nd and 3rd rounds of screening. This analysis indicated that compared to subjects with OLD who underwent screening with chest x-ray, LDCT screening resulted in a 37% reduction in mortality 5 years after the 2nd round of screening. This is a reflection of the proportion of early stage LCs diagnosed in each arm (73% in LDCT vs 52% in chest X-ray) who were candidates for “curative” resection therapy.12
These findings confirm and expand on previous studies. A recent analysis of the ACRIN cohort of the NLST revealed that the presence of spirometrically detected OLD increases the risk of LC and that screening for LC with LDCT results in a stage shift toward early stages without associated over diagnosis.3 Our analysis using the same cohort shows that screening with LDCT could also significantly decrease LC mortality in OLD patients, although this reduction only occurs in subjects diagnosed with lung cancer at the 2nd round of screening and beyond. What effect on mortality may accrue if annual screening were to be continued after the 3rd round can only be speculated on, but it is likely greater.
Previous studies have suggested a positive effect of LDCT screening on lung cancer mortality in subjects with OLD. De Torres et al.6 retrospectively compared 333 matched individuals (same age, gender, BMI, pack years’ history and smoking status) with mild to moderate COPD (GOLD spirometric stages 1 and 2) from two different cohorts: one, in which a baseline chest X-ray ruled out prevalent LC and was then followed with usual care (i.e., no screening), and another in which subjects underwent yearly LDTC screening. During the 31 months of follow up, 12 LC deaths occurred in the control group and 1 in the LDTC screening group (p<0.001). In the other large randomized, prospective study on LC screening, the NELSON screening trial,13 spirometry was performed in 1108 participants with only 437 having OLD, a much smaller dataset which will likely be underpowered to determine if LDCT can decrease LC mortality in those subjects.
Importantly, the present work showed that 108 individuals with OLD had to be screened to avoid one LC death, a much lower number than in the 218 individuals without OLD. This information could have important implications because it reinforces the message that patients with OLD are an excellent target for LC screening programs.
Unfortunately, no information is available on how many of the smokers with spirometrically diagnosed OLD included in this study, actually have “clinical” Chronic Obstructive Pulmonary Disease (COPD), i.e.: with symptoms in addition to an FEV1/FVC<0.70. We do know from previous analysis14 of the same cohort that 87% of these individuals with OLD did not have a previous diagnosis of COPD, asthma, bronchiectasis, chronic bronchitis, or emphysema. We also know that 80% of them in the previous study and 76% in the present were in GOLD spirometric stages 1 and 2. This is important since LC is one of the most important causes of death in patients with COPD in stages GOLD 1 and 2.15,16 Furthermore, having mild to moderate respiratory dysfunction usually allows for a pulmonary resection in the setting of early stage LC.12 If these results are reproduced in a clinical setting, the potential impact on patient's outcomes could be important. A specific study in this population is urgently needed.
There are several limitations in the present work. Firstly, the number of events (LC deaths) in the sample analyzed including all rounds of screening was underpowered to demonstrate a statistical significance. The trend was important (25% decrease in LC mortality, p=0.09) giving support to the complementary landmark analysis performed. Secondly, the fact that only a landmark analysis after the first year of follow up reached statistical significance, implies that only those that already had a LDCT or a chest X-ray at baseline and then underwent LC screening are the ones who were found to benefit. In daily clinical practice, this is not really a limitation, since most patients with OLD have either a LDCT or a chest X-ray for the evaluation of the disease and therefore could be included in screening programs like the present one. Finally, only pre-bronchodilator spirometry was performed and considering that the literature suggests this overestimates the diagnosis of airway obstruction by 20–25%,17 this could greatly impact on the correct diagnosis of OLD. However, the fact is that the presence of pre-bronchodilation OLD was associated with a trend of reduced lung cancer mortality in those who underwent screening.
In summary, this retrospective analysis of the prospectively recruited NLST-ACRIN database demonstrated that screening spirometrically confirmed OLD smokers for LC using LDCT, resulted in a trend to reduce lung cancer mortality. A study with a larger number of patients that includes COPD as a paramenter to take into account a priori, would be needed to confirm these findings.
Authors disclosureThe authors have reported the following: None declared (JPDT, JJZ, JPW, GB, DW and BRC).
Conflict of interestThe authors declare that they have no conflict of interest.