The SEPAR Spanish Bronchiectasis Registry (RIBRON) began as a platform for the collection of longitudinal data on patients with this disease. The objective of this study is to describe its operation and to analyze the characteristics of bronchiectasis patients according to sex.
MethodsA total of 1912 adult patients diagnosed with bronchiectasis in 43 centers were included between February 2015 and 2019. All patients had complete data consisting of at least 79 basic required variables, controlled by an external audit.
ResultsMean age was 67.6 (15.2) years; 63.9% were women. The most common symptom was productive cough (78.3%) which was mucopurulent-purulent in 45.9% of cases. The most common etiology was post-infectious (40.4%), while 18.5% were idiopathic. Pseudomonas aeruginosa was the most frequently isolated microorganism (40.4%), of which 25.6% were associated with chronic infection. The annual number of mild-to-moderate/severe exacerbations was 1.62 (1.9)/0.59 (1.3). Half of the patients (50%) presented with airflow obstruction (17% severe). The most frequent radiological localization was lower lobes. The average FACED/E-FACED/BSI values were 2.06 (1.7)/2.67 (2.2)/7.8 (4.5), respectively. Overall, 66.7% of patients were taking inhaled corticosteroids, 19.2% macrolides, and 19.5% inhaled antibiotics. Women presented a less severe profile than men in clinical and functional terms, and a similar infectious, radiological and therapeutic profile.
ConclusionsRIBRON represents an excellent map of the characteristics of bronchiectasis in our country. Two thirds of patients are women who presented lower disease severity as a specific characteristic.
El Registro Español de Bronquiectasias de la SEPAR (RIBRON) comenzó como una plataforma longitudinal de recogida de datos de pacientes con esta enfermedad. El objetivo del presente estudio es describir tanto su funcionamiento, como analizar las características de los pacientes con bronquiectasias según el sexo.
MétodosEntre febrero de 2015 y 2019, fueron incluidos 1.912 pacientes adultos diagnosticados de bronquiectasias procedentes de 43 centros. Todos los pacientes presentaron al menos datos completos de 79 variables básicas necesarias y controladas mediante una auditoria externa.
ResultadosEdad media fue de 67,6 años (15,2), el 63,9% mujeres. El síntoma más común fue la tos productiva en el 78,3%, que fue mucopurulenta-purulenta en el 45,9%. La etiología más frecuente fue la postinfecciosa en el 40,4%, siendo idiopáticas en el 18,5%. Pseudomonas aeruginosa fue el microorganismo más frecuentemente aislado con el 40,4%, el 25,6% en forma de infección crónica. El número anual de agudizaciones leve-moderadas/graves fue de 1,62 (1,9)/0,59 (1,3). El 50% de los pacientes presentaron obstrucción al flujo aéreo (el 17% grave). La localización más frecuente fueron los lóbulos inferiores. El valor medio del FACED /E-FACED/BSI fue de 2,06 (1,7)/2,67 (2,2)/7,8 (4,5), respectivamente. El 66,7% de los pacientes tomaban corticoides inhalados, el 19,2% macrólidos y el 19,5% antibióticos inhalados. Las mujeres presentaron un perfil de menor gravedad que los varones en términos clínico-funcionales y etiológicos, pero semejante perfil infeccioso, radiológico y terapéutico.
ConclusionesRIBRON representa un excelente mapa de las características de las bronquiectasias en nuestro país. Dos tercios de los pacientes son mujeres que presentaron unas características propias, de menor gravedad de la enfermedad.
Scientific knowledge of bronchiectasis has clearly grown rapidly over the past few years, as demostrated by the publication of various national and international guidelines,1–4 and the implementation of several registries that have accumulated large amounts of data from patients with this disease.5–10
The Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) has been a pioneer on both fronts. It achieved a world first with the publication of its bronchiectasis guidelines in 2008, which have been recently updated and have clearly contributed to improvements in the care and follow-up of our patients.11 It also set up the first national bronchiectasis registry (Historical Registry: 2002–2011) that accumulated information from more than 2,000 patients in 36 hospital, contributing to several important publications.12–14
In February 2015, the SEPAR Bronchiectasis Integrated Research Program (PII), under the auspices of Fundación Respira, implemented a new bronchiectasis registry that also included patient follow-up data in an attempt to obtain a more reliable analysis of the natural history of the disease over time. This initiative led to the birth of the ongoing Spanish Bronchiectasis Registry (RIBRON),15 which at the time of writing (July 2019) had already included more than 2,300 patients from 43 hospitals throughout Spain, making it, along with the US registry,7 one of the largest in the world. Given the wide participation by hospitals throughout the country, the analysis of these patient data is helping us paint an accurate picture of the general characteristics of bronchiectasis in Spain.
The main objective of this study was both to describe the peculiarities of the operation of the RIBRON registry and to analyze the main characteristics of the bronchiectasis patients included in the registry according to sex.
Materials and methodsRIBRON objectivesRIBRON is a centralized database of bronchiectasis patients from 43 hospitals located throughout Spain. The objectives of this record are:
- 1
To obtain information from bronchiectasis patients to improve the knowledge of the disease in our country.
- 2
To facilitate and promote multicenter and multidisciplinary research in bronchiectasis.
- 3
To identify groups of patients who may be candidates for future clinical trials.
- 4
To assess the follow-up of Spanish bronchiectasis clinical guidelines and recommendations in the attempt to standardize and improve patient management.
The inclusion criteria are as follows: patients over 18 years of age with bronchiectasis of any etiology (including cystic fibrosis [CF], although only non-CF bronchiectasis will be considered for this analysis). All patients should be diagnosed with chest high-resolution computed tomography (HRCT) and have a clinical picture consistent with the disease. Traction bronchiectasis was excluded. All ethics committees in the participating centers approved the registry, and all patients signed an informed consent to include their data in the registry. The recommendations of the Helsinki Declaration, good clinical practice guidelines, and currently applicable data protection laws were followed at all times. The registry is sponsored by SEPAR.
Data collectionData entry for each patient consists of a broad set of variables composed of 637 baseline and annual follow-up items, of which 79 were specified as basic or critical. These were defined as the minimum variables that, given their relevance, had to be entered for patients to be considered valid. These variables comprise general and anthropometric data, diagnostic variables, etiology and complementary etiological tests, disease history and comorbidities, clinical picture and physical examination, lung function, basic laboratory tests (in addition to those specific for etiological diagnosis), radiological diagnosis, microbiological data, exacerbations, complications, and treatments (pharmacological and non-pharmacological) both in stable phase and during exacerbations. In addition to these variables, an annual follow-up section is completed which lists any new circumstances that have emerged since the last visit, encompassing all the aspects mentioned above.
RIBRON quality controlA company not affiliated with RIBRON and independent from researchers and SEPAR is responsible for data quality control. To this end, various measures have been established:
- 1
In all variables, values entered outside the reasonable preset ranges trigger an alert. In this case, the the investigator is notified that the datum cannot be entered.
- 2
If a confusing or conflicting data point is detected, the company contacts the investigator to certify the authenticity of the entry.
- 3
An annual database sweep is performed for abnormal data or missing data. The corresponding researchers are informed of the findings in an external audit that is recorded, and a report is generated and forwarded to the registry scientific committee
- 4
Validation is performed individually for each patient.
- 5
A data dump is performed annually in SPSS® or Excel® format and the content is purged by a professional statistician.
- 6
The company ensures the confidentiality of the data according to applicable legislation.
This study analyzed the characteristics of 1,912 patients from 43 hospitals throughout Spain in terms of general and anthropometric data, diagnostic variables, etiology and complementary etiological tests, disease history and comorbidities, clinical picture and physical examination, lung function, radiological diagnosis, microbiological data, exacerbations, and treatments (pharmacological and non-pharmacological). Variables are tabulated by mean (standard deviation) for variables with normal distribution, median (interquartile range) for non-normal distribution, and by percentage of total for qualitative or dichotomous variables. A Mann-Whitney U/Student’s t test or Chi-squared test (depending on the type and distribution of variables) was used to analyze the differential characteristics between groups according to sex. A p-value of <0.05 was considered statistically significant. The data were analyzed using the SPSS® statistical package version 21.0.
ResultsAs of February 2019, a total of 2,115 patients had been included, 29 of whom had pending data, 47 were invalid, and 127 were diagnosed with CF. Therefore, 1,912 patients diagnosed with bronchiectasis in 43 hospitals were analyzed in this study (Fig. 1).
Demographic and anthropometric dataMean age was 67.6 years (15.2), 43.8% were over 70 years of age, and there was a predominance of women (63.9%). In total, 96.7% were Caucasian. The diagnostic delay was 6.2 years (12.1). Body mass index (BMI) was 25.6 kg/m2 (4.8) (Table 1).
Demographic data, disease history and main clinical characteristics of patients.
| N = 1,912 patients, mean (SD) or percentage | |
|---|---|
| Demographic data | |
| Age, years | 67.6 (15.2) |
| Percentage of patients > 70 years old, % | 43.8 |
| Sex, % women | 63.9 |
| Race, % Caucasian | 96.7 |
| BMI, kg/m2 | 25.6 (4.8) |
| Diagnostic delay, years | 6.2 (12.1) |
| Disease history | |
| Nasal rhinosinusitis-polyposis, % | 27.7 |
| Smoking habit, pack-years | 31.4 (27.6) |
| Never smokers, % | 58.4 |
| Former smokers, % | 33.3 |
| Active smokers, % | 8.3 |
| Alcohol habit, g/day | 35.8 (30.8) |
| Cardiovascular disease, % | 17.4 |
| Coronary artery disease, % | 4 |
| Heart failure, % | 7.2 |
| Peripheral artery disease, % | 2.7 |
| Cor pulmonale, % | 1.2 |
| Cerebrovascular disease, % | 3.6 |
| Diabetes mellitus, % | 9.8 |
| Renal failure, % | 3.2 |
| Anxiety/depression, %/% | 7.4/8.4 |
| Use of at least 1 psychoactive drug, % | 34.9 |
| Tumor history, % | 7.2 |
| Charlson index | 1.83 (1.46) |
| Clinical characteristics and physical examination | |
| Habitual cough (every day or almost every day), % | 78.3 |
| Expectoration, quantity | |
| Occasional, % | 16 |
| <20 cc/day, % | 26.1 |
| 20–50 cc/day, % | 20.3 |
| 50–100 cc/day, % | 6.4 |
| >100 cc/day, % | 0.8 |
| Expectoration (usual color) | |
| Mucoid, % | 53 |
| Mucopurulent, % | 33.6 |
| Purulent, % | 12.3 |
| Bloody, % | 1.1 |
| Dyspnea (mMRC scale) | |
| -/IV, % | 39.9 |
| I/IV, % | 30.9 |
| II/IV, % | 24.1 |
| III/IV, % | 4.3 |
| IV/V, % | 0.8 |
Most patients had never smoked (58.4%), and did not consume alcohol (58%). Among smokers and former smokers, the number of pack-years was 31.4 (27.6); 8.3% were active smokers and 33.3% were former smokers. The mean Charlson index was 1.83 (1.46).
The most commonly reported disease history was nasal rhinosinusitis/polyposis, which was present in 27.7%, followed by cardiovascular disease (17.4% of patients had at least one previous cardiovascular event), and tumor disease (7.2%). Between 7.4% and 8.4% of patients were diagnosed with anxiety or depression, but more than a third of the overall group of study patients (34.9%) were receiving at least one psychoactive drug (Table 1).
Clinical dataThe most frequent symptom was cough (78.3%) with expectoration (69.6%). Overall, 29.2% of patients had at least grade II/IV dyspnea (mMRC) (Table 1).
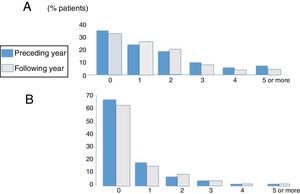
ExacerbationsFig. 2 shows the distribution of patients according to the number and severity of exacerbations during the year before and the year after inclusion in the registry. A mean of 1.62 (1.9) mild-moderate exacerbations and 0.59 severe exacerbations (need for hospitalization or intravenous antibiotic therapy in hospital or at home due to exacerbation) occurred in the year before inclusion,1,3 and 1.551,7 and 0.571,2 in the year following inclusion, respectively. No significant differences were found in the number or severity of exacerbations between those occurring in the year before and the year after inclusion in the registry. Overall, 57.4%, 19.5%, and 10.9% of patients had at least 1, 2, or 3 exacerbations per year, while 13.2% had 1 or more hospital stays per year. Using a definition of an exacerbator as a patient with at least 2 exacerbations per year or 1 hospitalization per year 16 22.4% of the series could be considered as exacerbators.
Etiological dataValid etiological data were available from 99.4% of patients. Table 2 lists the most common etiologies by groups. The most frequent etiology was post-infectious disease (40.4%), while 18.5% of patients presented bronchiectasis defined as idiopathic.
Etiology of bronchiectasis in the RIBRON registry.
| Etiology | Percentage |
|---|---|
| Idiopathic | 18.5 |
| Post-infectious | 40.4 |
| Tuberculosis | 13.5 |
| Associated with COPD | 10.9 |
| Associated with asthma | 7.8 |
| Immunodeficiencies | 4.2 |
| Genetic diseases | 4.2 |
| Systemic diseases | 4 |
| Inflammatory pneumonitis | 1.7 |
| Allergic bronchopulmonary aspergillosis | 0.9 |
| Congenital malformations | 0.7 |
| Inflammatory bowel disease | 0.8 |
| Bronchiolitis obliterans | 0.6 |
| Bronchial obstruction | 0.3 |
| Hyperimmune response | 0.3 |
| Vasculitis | 0.2 |
| Other | 4.5 |
COPD: chronic obstructive pulmonary disease.
Mean baseline FEV1 was 1.86 l (0.8) (73.9% predicted), while mean forced vital capacity (FVC) was 2.66 (0.9) (83.8% predicted). In total, 53.1% had airflow obstruction defined as an FEV1/FVC ratio < 0.7, while 17% had severe airflow obstruction (FEV1 < 50%), and 19.8% of patients had a positive bronchodilator test. Six-minute walk test data were available from 131 patients who walked an average of 425.3 m (114). On average, alveolar volume-corrected carbon monoxide diffusion values (DLCO/VA) were found to be within normal parameters: 81%. Mean arterial blood gases showed moderate hypoxemia in blood gases (pO2: 66 mmHg [17.5] and pCO2: 39.7 mmHg [6.7]). Data were available from 355 patients with lung volumes showing a mean total lung capacity (TLC) of 6.43 l (3.1) (102% predicted) and a residual volume (RV) of 3.78 l (11.5) (138% predicted).
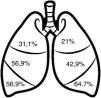
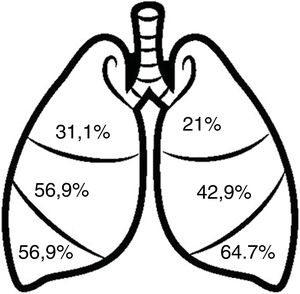
Radiological dataFig. 3 shows the distribution of bronchiectasis by pulmonary lobes. The most frequent locations were the lower lobes (64.7–56.9%) and the middle lobe (56.9%). Bronchiectasis in more than 2 pulmonary lobes was observed in 48.2%, 90.9% of which were cylindrical. Varicose bronchiectasis was detected in 26.4% of the patients and cystic bronchiectasis in 21%. In addition, 19.4% presented radiological images consistent with bronchiolitis (“tree-in-bud” image).
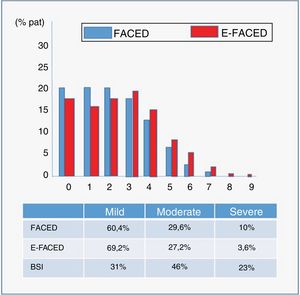
Severity scoresMean FACED (17) and E-FACED (18) scores were 2.06 (1.69) and 2.67 (2.17), respectively, while the Bronchiectasis Severity Index (BSI) (19) was 7.8 (4.5). Fig. 4 shows the percentage by severity (mild, moderate, or severe) of the 3 multidimensional scores, and the graphical distribution of FACED and E-FED scores.
Microbiological dataTable 3 summarizes the main microbiological characteristics of bronchiectasis patients. Overall, 38.5% had initial colonization, 16.7% intermittent, and 44.8% chronic bronchial infection. The most common potentially pathogenic microorganism (PPM) involved (both in single isolations and in the form of chronic bronchial infection [CBI]) was Pseudomonas aeruginosa (P. aeruginosa) with 40.4% and 25.6% of patients, respectively. Only atypical mycobacteria were isolated in 26 patients, the most frequent being Mycobacterium avium complex.
Isolates and chronic bronchial infection by potentially pathogenic microorganisms.
| Microorganism | Isolates | Chronic bronchial infection |
|---|---|---|
| Bacteria | ||
| P. aeruginosa (mucoid), % (%) | 40.4 (11.2) | 25.6 (8) |
| H. influenzae, % | 18.9 | 14.1 |
| S. aureus (MR),% (%) | 7.6 (0.6) | 7.5 (0.3) |
| M. catarrhalis, % | 5.4 | 4.7 |
| S. pneumoniae, % | 5.1 | 5.2 |
| Enterobacteria, % | 5.3 | 6.1 |
| Klebsiella spp., % | 1.9 | 2.1 |
| S. maltophilia,% | 2.4 | 1.5 |
| B. cepacea, % | 0.1 | 0.3 |
| Acinetobacter spp., % | 0.8 | 0.3 |
| A. xylosoxidans, % | 1.4 | 1.5 |
| Other, % | 4 | 3.3 |
| Mycobacteria | ||
| M. tuberculosis, % | 0.1 | |
| M. avium, % | 0.7 | |
| M. abscessus, % | 0.2 | |
| M. kansasii, % | 0.2 | |
| M. fortuitum, % | 0.1 | |
| M. xenopi, % | 0.1 | |
| Other, % | 0.2 | |
| Fungi | ||
| Candida albicans, % | 2.4 | |
| Aspergillus fumigatus, % | 0.7 | |
| Aspergillus niger, % | 0.2 | |
| Penicillium, % | 0.2 | |
| Other, % | 0.5 | |
A total of 75% of patients were receiving chronic corticosteroids, of which 87% were inhaled (66.7% of all patients); 77% of patients were taking some type of bronchodilator treatment (67% short-acting beta 2; 68% long-acting beta 2; and 44% anticholinergics); 60.2% of patients followed a respiratory physiotherapy program, with manual secretion drainage techniques in 69% of cases; 8.2% used mucolytics and 2.5% hypertonic serum, mainly 7%; 7.4% of patients received home oxygen therapy, while 0.9% required non-invasive mechanical ventilation. Finally, 19.2% received chronic macrolides (93% azithromycin) and 19.5% inhaled antibiotics (78% of which were nebulized sodium colistimethate).
Differentiation by sexTable 4 summarizes the general characteristics of the patients by sex. Women had a very different disease profile from men, with a lower age, lower BMI, fewer symptoms, fewer comorbidities, and more mental disorders (anxiety/depression and taking psychoactive drugs). Bronchiectasis among women was due more to idiopathic forms, post-infectious disease, and asthma, and less to COPD. Disease severity was lower, they had fewer admissions for exacerbations, and their lung function was better. However, there were no differences in the number of mild-moderate exacerbations, the presence of CBI caused by P. aeruginosa, antibiotic or anti-inflammatory treatment of the infection, or radiological extension of the bronchiectasis.
Comparison of bronchiectasis characteristics by sex.
| Variable | Men (n = 691) | Women (n = 1,221) | p-value |
|---|---|---|---|
| Age | 68.3 (16) | 67.1 (15) | 0.039 |
| Age >70 | 49.9 | 40.4 | <0.0001 |
| BMI, kg/m2 | 26.6 (4.2) | 25.1 (4.9) | <0.0001 |
| Dyspnea | 1.2 (0.4) | 1.1 (0.3) | <0.0001 |
| Charlson index | 2.2 (1.8) | 1.6 (1.2) | <0.0001 |
| Anxiety | 4.1 | 11 | <0.0001 |
| Depression | 5.1 | 10.2 | <0.0001 |
| Idiopathic | 13.7 | 21.3 | <0.0001 |
| Post-infectious | 34.9 | 43.5 | <0.0001 |
| COPD | 23.2 | 4.01 | <0.0001 |
| Asthma | 5.6 | 9 | <0.0001 |
| FACED | 2.4 (1.8) | 1.9 (1.6) | <0.0001 |
| E-FACED | 3.1 (2.3) | 2.4 (2) | <0.0001 |
| BSI | 8.7 (4.8) | 6.9 (4.5) | <0.0001 |
| CBI-PA | 23.8 | 22.3 | 0.43 |
| Affected lobes | 2.76 (1.4) | 2.76 (1.4) | 0.95 |
| Exacerbations | 1.5 (1.8) | 1.7 (1.9) | 0.14 |
| Hospitalization due to exacerbation | 0.73 (1.5) | 0.51 (1.9) | 0.0001 |
| FEV1, % | 68.1 (23) | 77.2 (24.1) | <0.0001 |
| FVC, % | 78.8 (19.2) | 86.5 (22.9) | <0.0001 |
| FEV1/FVC, % | 65.5 (15) | 71.1 (12.7) | <0.0001 |
| Macrolides | 21.3 | 18.1 | 0.089 |
| Inhaled antibiotics | 20.4 | 18.5 | 0.13 |
BMI: body mass index; BSI: Bronchiectasis Severity Index; CBI-PA: chronic bronchial infection caused by Pseudomonas aeruginosa; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Variables tabulated by mean (standard deviation) for quantitative values and percentage of total for qualitative values.
This study is the first communication of data from the RIBRON (Spanish Bronchiectasis Registry) which has included more than 2,000 evaluable patients in 43 hospitals. According to the results of this study, almost two-thirds of patients with bronchiectasis are women. They have a very different profile from men, with less severe disease in clinical-functional and etiological terms (higher percentage of idiopathic forms, asthma and post-infectious disease), but their infectious profile (similar number of P. aeruginosa isolates and CBIs), and radiological and therapeutic profiles are similar. Finally, the results of this registry show a high percentage in Spain of isolates and CBI caused by PPM, especially P. aeruginosa, which is related to a significant number of patients receiving long-term antibiotic treatment, especially in inhaled preparations with anti-inflammatory effects (macrolides).
Over the past few years, several national and international registries conducted in different countries have accumulated a significant amount of information about bronchiectasis.5,15 Of these, the US7 and Australian6 registries have published overall results on 589 and 1,826 patients, respectively, while other registries such as the Italian (IRENE/IRIDE),8,9 German (PROGNOSIS)5 or European (EMBARC)10 projects have so far only published data on methodology, along with some specific data.
Patients in the Spanish registry are similar to those in the US and Australian registry in terms of average age and the percentage of women (even higher in the other 2 registries). More similarities include the predominant site as the lower and middle lobes, the principal clinical characteristics, comorbidities, and number and severity of exacerbations.
However, there are some differentiating features. In the Spanish registry, the number of isolates and CBIs caused by different microorganisms, especially P. aeruginosa, is higher than in the US or Australia. These data coincide with those contributed by different microbiological agencies that emphasize the existence in Europe of a north-south trend of growing P. aeruginosa isolation and resistance rates, requiring aggressive treatment in these countries.17 This could explain the higher rate of long-term antibiotic and macrolide use in Spain18 compared with northern European countries. The microbiological situation is similar (if not worse) in Latin America and China, where the percentage of patients with P. aeruginosa isolates or CBIs far exceeds 40%.19,20 In the US, on the other hand, the prevalence of non-tuberculous mycobacteria infection is over 66%, which is unusually high,7 whereas in Spain it is an unusual finding, although one that is gaining importance.21
It is important to note that the percentage of idiopathic bronchiectasis is significantly lower in Spain compared to other registries. This may be because guidelines have been in place in Spain for more than a decade, and this might have contributed to a better etiological diagnosis, but it cannot be ruled out that some bronchiectasis labeled as post-infectious disease may not have been generated by infection (especially those associated with viruses and other childhood infections), given the difficulty of determining a cause-effect relationship between the two entities.
The significant use of inhaled corticosteroids (66.7% of patients) is interesting, especially when we consider that only 7.8% of patients had asthma and 0.9% had allergic bronchopulmonary aspergillosis, and despite the fact that none of the national1,2 or international guidelines3,4 recommend their use in patients with bronchiectasis. This excessive use of inhaled corticosteroids is not unique to our country. Indeed, the American registry recorded a rate of 39%,7 while the proportion is higher than 50% in many of the European countries participating in the European EMBARC registry (unpublished data). This excessive use of inhaled corticosteroids in bronchiectasis is clearly an extremely important issue that must be addressed in the near future: patients who could benefit most from this treatment must be more precisely identified, and the existence of biomarkers that predict a good (or bad) response or the future occurrence of side effects from this treatment must be investigated.22
Finally, our results show striking differences among bronchiectasis patients according to sex. As in most studies, and despite the increase in the percentage of patients with COPD-bronchiectasis overlap (most common in men),23,24 almost two-thirds of the patients in our registry were women. Our female patients had less serious forms of the disease in clinical and functional terms, and they presented less serious exacerbations, as can be seen in Table 4, despite having similar infectious and radiological profiles. The reduced functional involvement and lower number of hospitalizations meant that women scored lower in all multidimensional severity and prognosis scales,25–27 although the diagnostic delay was greater in women than in men.12 Finally, it is important to emphasize the higher percentage of anxiety and depression and use of psychoactive drugs among women. Although this has been observed in various chronic diseases, stricter monitoring of these comorbidities is clearly needed, since they are closely related to the quality of life of patients, and also with bronchiectasis.28
The limitations of the RIBRON registry must be mentioned: only 79 of the 637 variables were mandatory, so the percentages of missing data for variables such as quality of life or some specific complementary tests vary.
In conclusion, this analysis of the Spanish Bronchiectasis Registry (RIBRON) that includes nearly 2,000 patients paints an accurate picture of the characteristics of bronchiectasis in our country, and may be the basis for the implementation of multiple studies on this disease. Two-thirds of patients are women, and their characteristics, especially clinical features, differ widely from those of men, so sex-specific studies are important in determining whether women need more personalized treatment and monitoring.
Members of the Spanish RIBRON GroupAnnie Navarro Rolon, Hospital Mutua Terrassa, Barcelona; Patricia Minguez, Hospital Puerta de Hierro, Madrid; Rosanel Amaro, Hospital Clinic, Barcelona; Angela Cervera, Hospital General, Valencia; Marina Blanco, Hospital A Coruña, Corunna; Ainhoa Gomez, Hospital Cruces, Bilbao, Biscay; Eleuterio Llorca, Hospital de Elda, Alicante; Alicia Padilla, Hospital de Marbella, Malaga; Edmundo Rosales, Hospital General de Cataluña, Barcelona; Laura Carrasco, Hospital Virgen del Rocio, Seville and Marcelo Razquin, Fundació Hospital de Nens, Barcelona.
FundingThe RIBRON register is funded by Zambon SA laboratories, which has not been involved in the design, data collection or interpretation, or the writing of this manuscript.
Conflict of interestsThe authors state that they have no conflict of interests.