In a position paper published in 1995, the American Thoracic Society (ATS) officially recognized that the exacerbation of chronic obstructive pulmonary disease (COPD) was difficult to define, and that its pathogenesis was still unclear. At the time, the society already mooted the need for a standardized definition that could be universally accepted and useful for clinicians, researchers, and other health providers.1 Five years later, responding to that challenge, a group of experts led by Dr. Rodríguez-Roisin2 defined exacerbation as “a sustained worsening of the patient's condition, from the stable state and beyond normal day-to-day variations, that is acute in onset and necessitates a change in regular medication in a patient with underlying COPD”. It has been 20 years since that initial proposal, and significant advances have been made in the knowledge of COPD, which is now understood as a complex and heterogeneous syndrome requiring an increasingly personalized approach.3 However, the concept, diagnosis, and management of exacerbations have hardly changed. We continue to indiscriminately use short-acting bronchodilators, systemic steroids, and antibiotics with poor outcomes. Therapeutic failures are common, the 30-day readmission rate is 20%, and in-hospital mortality is 5%, increasing to 11% at 3 months. Furthermore, a considerable proportion of patients have persistent exacerbations, we do not have a good classification system, we do not use generalized predictive risk models, admission criteria are not standardized, and clinical trials still define exacerbation or severity based on resource use.4–7 As a result, scientific results are very heterogeneous and progress is inconsistent.
The origin of this confusion lies in the construction of the definition itself, which fails to identify the underlying biological mechanism, does not recognize the heterogeneity of the clinical picture and, being symptom-based, is very unspecific. Many other concomitant diseases (comorbidities) can also cause the same worsening of symptoms in COPD patients. Heart failure, arrhythmias, ischemic heart disease, anxiety, pulmonary embolism, and pneumonia are diseases that often occur in COPD and present with increased respiratory symptoms. However, by consensus, they are not considered COPD exacerbations. A comprehensive differential diagnosis is needed to rule out these other diseases, but this is not always easy. Pneumonia, for example, is an entity that is typically distinct from exacerbation due to the presence of infiltrates in the pulmonary parenchyma. However, the difference between the two entities is very subtle. Up to one third of COPD patients have infiltrates on computed tomography that are not visible on simple chest X-ray.8 These patients are classified and categorized as COPD exacerbations when they are actually pneumonia. Are they different entities or phases of the same disease? The pulmonary microbiome is practically identical, causative factors are very similar, and clinical expression, prognostic impact, and treatment are also similar.8,9 A similar situation occurs with heart failure, ischemic heart disease, and pulmonary embolism, which often overlap with the exacerbation itself.10–12 The direct consequence of all this is confusion, heterogeneous clinical outcomes, scant progress in understanding the underlying mechanisms, and barriers to accessing personalized treatment. A change of strategy is therefore urgent. We need a new definition derived from more objective and specific criteria that, even at the risk of losing some sensitivity, also takes into account the variety of presentations, helps identify biological endotypes, and offers a direct pathway to precision medicine.
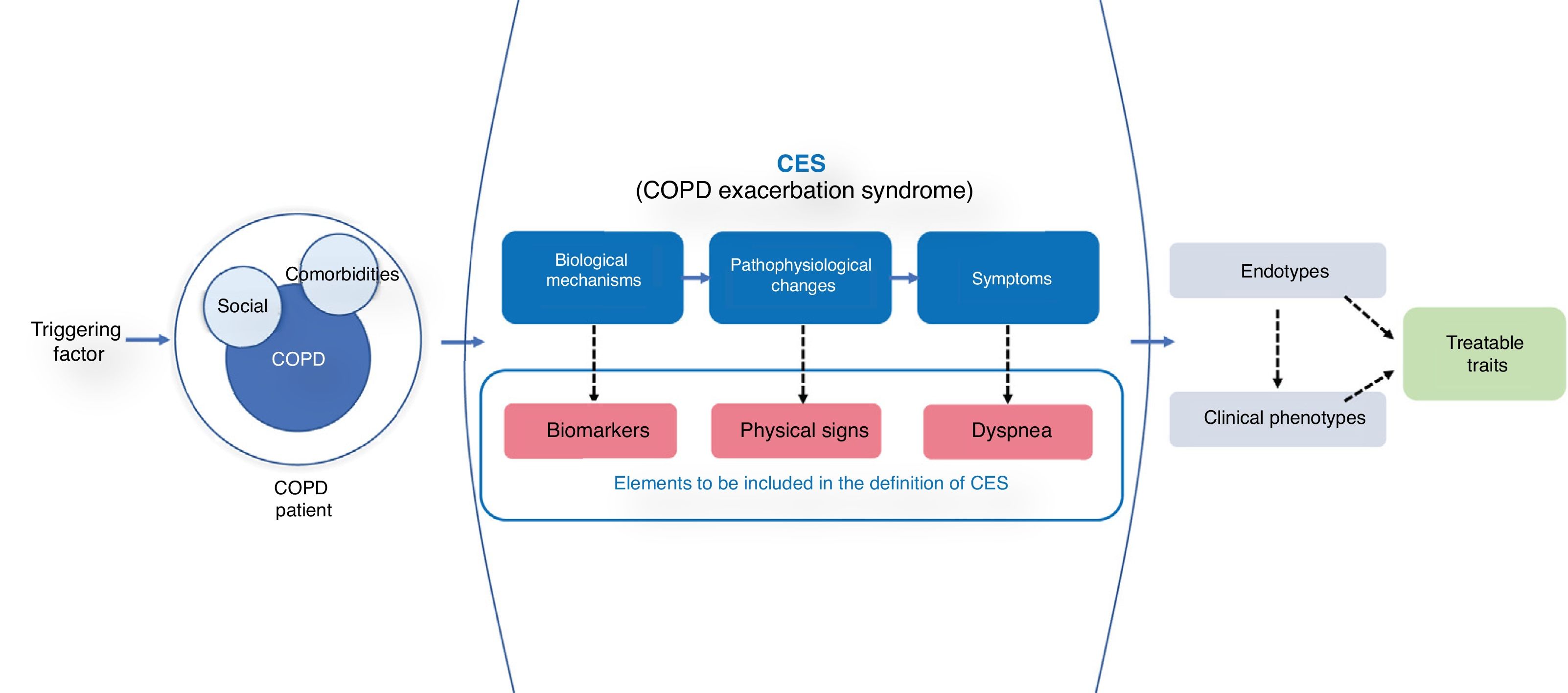
A good reference model is acute coronary disease, the current definition of which is based on 3 essential parameters: 1) a syndrome-based approach (acute coronary syndrome); 2) a guiding symptom (chest pain); and 3) objective variables with underlying biological mechanisms (electrocardiographic alterations and biomarkers).13 Using a syndrome-based approach to what we might call COPD exacerbation syndrome (CES) would allow us to take into account all other diseases that can produce similar symptoms that are very hard to differentiate. The advantage of this approach is that differentiated and/or combined clinical endotypes or phenotypes that may potentially have different outcomes and specific therapeutic approaches are taken into account (Fig. 1).
The diagram shows the concept of CES. The presence of a triggering factor in a COPD patient (with its social determinants and comorbidity) leads to the appearance of a CES syndrome, where worsening dyspnea is the final manifestation of various biological and pathophysiological mechanisms. Because of the heterogeneity of symptoms, different biological endotypes and various phenotypic presentations susceptible to specific treatments can be expressed.
Worsening dyspnea emerges as the guiding symptom of CES. Expiratory airflow limitation and dynamic hyperinflation have been described as the main pathophysiological mechanisms, although cardiovascular, muscle, and psychosocial disorders may also be involved.14 Changes in respiratory rate, heart rate and/or gas exchange are also an objective manifestation of underlying pathophysiological changes, so analysis of these parameters may be helpful.
Finally, we need to incorporate biomarkers associated with the underlying biological alteration that help identify and categorize exacerbations and guide treatment. Various, largely unsuccessful, attempts have been made to identify a reference biomarker for diagnosis.15 However, the simultaneous use of different biomarkers may open new avenues. In a multilevel analysis, Noell et al.16 found that the best biomarker panel to define exacerbation was the combination of circulating neutrophils, C-reactive protein, and dyspnea (area under the curve = 0.97). Peripheral eosinophilia, procalcitonin, D-dimer or cardiovascular biomarkers such as troponin or N-terminal pro-brain natriuretic peptide (NT-pro-BNP) are some examples of other biomarkers that may be helpful in identifying traits that guide personalization of treatment,17 although none of them has been shown to be sufficiently specific to the disease. In contrast, some alveolar mediators, such as surfactant protein D, may be useful during COPD exacerbations.18
In short, a quarter of a century after the ATS recommendation, we need to change the paradigm and redefine exacerbation by incorporating elements that help improve the specificity of the clinical picture, without losing sensitivity. A syndrome-based approach may better address the heterogeneity of the process. Dyspnea emerges as the guiding symptom. However, given its subjective nature, it would be desirable to include some objective parameters associated with the pathophysiology of the episode, along with biomarkers that help to profile the underlying endotype. Only if we can improve the specificity of the picture will we be able to deepen our understanding of its biological mechanisms, customize treatments, and ultimately mitigate the negative consequences that these episodes generate.
Conflict of interestsJuan José Soler-Cataluña has received speaker fees from AstraZeneca, Boehringer Ingelheim, Bial, Ferrer, Laboratorio Esteve, Menarini, Mundipharma, Novartis, Rovi, and TEVA; consultancy fees from AirLiquide, AstraZeneca, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, Laboratorios Esteve, Mundipharma and Novartis, and research assistance from GlaxoSmithKline, Chesi, and Boehringer Ingelheim.
Cristina Miralles Saavedra states that she has no conflict of interests.
Please cite this article as: Soler-Cataluña JJ, Miralles C. Hacia el síndrome de agudización en la EPOC: un cambio de paradigma. Arch Bronconeumol. 2021;57:246–248.