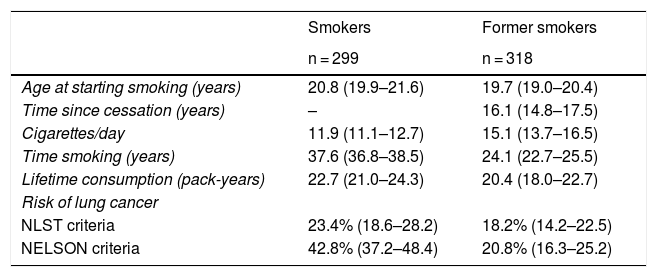Lung cancer mortality is increasing in women. In Spain, estimates suggest that lung cancer mortality may soon surpass breast cancer mortality, the main cause of cancer mortality among women. The aim of this study was to estimate the proportion of women at high risk of developing lung cancer in a group of participants in a population-based breast cancer screening program.
MethodsCross-sectional study in a sample of women who participated in a population-based breast cancer screening program in 2016 in Hospitalet de Llobregat n = 1,601. High risk of lung cancer was defined according to the inclusion criteria of the National Lung Screening Trial (NLST) and the Dutch-Belgian randomized lung cancer screening trial (NELSON).
ResultsAround 20% of smokers according to NLST and 40% of smokers according to NELSON criteria, and around 20% of former smokers according to both criteria, are at high risk of developing lung cancer. A positive and statistically significant trend is observed between the proportion of women at high risk and nicotine dependence measured with the brief Fagerström Test.
ConclusionsA high proportion of participants in this breast cancer screening program have a high risk of developing lung cancer and would be eligible to participate in a lung cancer screening program. Population-based breast cancer screening programs may be useful to implement lung cancer primary prevention activities.
La mortalidad por cáncer de pulmón está aumentando en mujeres. Se ha proyectado que en España pueda superar a la mortalidad por cáncer de mama, la principal causa de mortalidad por cáncer en mujeres, en pocos años. El objetivo de este estudio es estimar la proporción de mujeres que presentan alto riesgo de desarrollar cáncer de pulmón en un grupo de participantes en un cribado poblacional de cáncer de mama.
MétodosEstudio transversal de una muestra de mujeres que participaron en un cribado poblacional de cáncer de mama en el año 2016 en Hospitalet de Llobregat (n = 1.601). El riesgo elevado de cáncer de pulmón se definió según los criterios del National Lung Screening Trial (NLST) y del Dutch-Belgian randomised lung cancer screening trial (NELSON).
ResultadosAlrededor de un 20% y un 40% de fumadoras según los criterios NLST y NELSON, respectivamente, y alrededor de un 20% de exfumadoras según ambos criterios, presentan un alto riesgo de desarrollar cáncer de pulmón. Se observa una tendencia positiva y estadísticamente significativa entre la proporción de mujeres que presentan alto riesgo y la dependencia a la nicotina medida por el Test de Fagerström breve.
ConclusiónUna alta proporción de participantes en este cribado de cáncer de mama presenta un riesgo elevado de desarrollar cáncer de pulmón y sería elegible para participar en un programa de cribado de cáncer de pulmón. Los cribados poblacionales de cáncer de mama pueden ser útiles para implementar estrategias de prevención primaria de cáncer de pulmón.
Smoking is the second worldwide risk factor for mortality after exposure to contamination,1 and is considered a health inequity factor that affects socio-economically disadvantaged populations.2 It is estimated that around 50% of smokers die from their habit.3 In addition to its high mortality, smoking is associated with significant morbidity and carries a heavy burden in terms of costs and use of health services.4
Smoking is associated with different diseases, including several types of cancer, such as bladder and lung malignancies.4 One of the most lethal is lung cancer,5 with a survival rate in Spain of 37.7% 1 year after diagnosis, 14.9% at 3 years, and 10.7% at 5 years.6 These low survival rates are often the result of late detection at stages III-IV.7 Among women, mortality from lung cancer is on the rise, and is forecast to exceed that of breast cancer in the next few years in various mid-to-high income countries,8 including Spain,9 a consequence in this case of the increase in lung cancer incidence in women from 7% between 1993 and 1997 to 11.2% between 2003 and 2007.10
The particular characteristics of lung cancer mean that primary prevention activities (smoking cessation and prevention of smoking) are essential to reduce the incidence and, consequently, the mortality of this disease. Moreover, the positive consequences of stopping smoking extend beyond the time of diagnosis of lung cancer, because even after an early-stage diagnosis, quitting smoking can reduce the risk of death by up to half,11 with added benefits such as reduced pain12 and better functional status.13 In this respect, population screening has been described as a “teachable moment”, an opportunity to implement primary prevention activities and encourage healthy lifestyle habits.14 Furthermore, participation in breast cancer screening is generally high,15 and this may help include a greater number of women in such prevention activities.
Several studies have been carried out in recent years aimed at analyzing the reduction in lung cancer mortality and all-cause mortality conferred by the use of low-dose CT16–18 screening (secondary prevention). The ultimate goal of these studies is to assess the potential utility of selective screening programs in healthcare systems among adult smokers and former smokers with a history of medium-to-high cumulative consumption. The most important in terms of design was the National Lung Screening Trial (NLST) in the United States, which showed a reduction in lung cancer mortality of 20% and all-cause mortality of 6.7% compared to screening with chest X-rays.16 These results led various entities, such as the US Preventive Services Task Force,19 to recommend low-dose computed tomography (CT), that was eventually implemented for high-risk groups in the United States.20 Various studies have also been conducted in the European Union (EU), where lung cancer screening has not yet been implemented. The randomized Dutch-Belgian lung cancer screening trial study, the NELSON trial, is notable for its sample size.18 Preliminary reports showed a 26% (p = 0.003) and 39% (p = 0.0543) reduction in mortality from lung cancer after 10 years of follow-up in men and women, respectively.21 Variables as inclusion criteria for both trials were age and cumulative consumption of cigarettes in pack-years, an approach described by other authors as simplified eligibility criteria as opposed to risk prediction models that take into account additional variables (for example, comorbidities, and exposure to other agents).22 In terms of cost-effectiveness, it has been estimated that for the cost of 1 lung cancer screening procedure, 20 smoking cessation interventions could be funded.23
For these reasons, and given the increase in lung cancer mortality in women, the aim of this study was to estimate the proportion of participants in a population breast cancer screening program that present a high risk of developing lung cancer according to the inclusion criteria in the NLST and NELSON studies.
MethodsDesignThis was a cross-sectional study in a series n = 1,601 of women participating in a population breast cancer screening program. The study was conducted between May and July 2016 in the Catalan Institute of Oncology ICO, L’Hospitalet de Llobregat. Two technicians individually performed the mammograms for the population breast cancer screening program. Once completed, the women were asked to consent to participate in a study on tobacco consumption patterns and dependency. Those that agreed completed a brief face-to-face questionnaire consisting of up to 16 questions, depending on whether they were never smokers, former smokers, or current smokers, plus two additional questions on their level of education and recontact details (total of 18 questions).
Statistical analysisA descriptive analysis was performed to define the consumption patterns of women smokers and former smokers from the self-declared information in the questionnaire. For smokers, we calculated the mean and 95% confidence interval (95% CI) of age at starting smoking, cigarettes smoked per day, and length of time smoking. Cumulative consumption over the subject’s lifetime was then estimated in pack-years from the number of cigarettes smoked per day divided by 20 (cigarettes per pack) multiplied by years of smoking. Mean and 95% CI for the variable cumulative consumption were calculated. In addition to the above, mean and 95% CI of the time in years since cessation were calculated for former smokers.
We estimated the proportion of the total number of women smokers and former smokers who currently presented a high risk of developing lung cancer according to NLST and NELSON criteria, defined as: (i) age between 55 and 74 years, with consumption of at least 30 pack-years and, in the case of former smokers, having quit in the last 15 years, according to NLST criteria16; and (ii) age between 50 and 75 years, with at least 25 years smoking more than 15 cigarettes a day or at least 30 years smoking more than 10 cigarettes a day and, in the case of former smokers, having quit in the last 10 years, according to NELSON criteria.18 To calculate cumulative historical consumption, the last reported use was taken as a constant from the date of starting smoking. We also estimated the proportions of women smokers and former smokers presenting a high risk stratifyingby the variables age (50–54 years, 55−59 years, 60–64 years, and 65–69 years) and screening round in which they were participating (first or second, third or fourth, fifth or sixth, seventh or eighth, and ninth or tenth) from anonymized information collected in the registry; nicotine dependence classified by the brief Fagerström test24 as low (0–2 points), medium (3–4 points), and high (5–6 points); and stage of change25 (precontemplation, contemplation and preparation). The statistical program used was SPSS v.21.
ResultsMean age of the participants was 61 years (standard deviation = 4.9). Overall, 18.7% stated that they were smokers and 19.9% former smokers. Around 73% had no studies or primary level studies only. The percentage of participation in the screening program in the study months was 75.5%, 78.4% and 70.9% for May, June and July respectively, while the response rate for the survey for the total number of participants in the screening was 95.5%, 90.1%, and 77.0% for the same months.
Table 1 shows the pattern of tobacco consumption in women smokers and former smokers and the proportion of women with a high risk of developing lung cancer. According to the NLST criteria, 23.4% (95% CI 18.6–28.2) of smokers and 18.2% (95% CI 14.2–22.5) of former smokers have a high risk of developing lung cancer. According to the NELSON criteria, 42.8% (95% CI 37.2–48.4) of smokers and 20.8% (95% CI 16.3–25.2) of former smokers have a high risk of developing lung cancer. On the basis of these proportions, and taking into account that approximately 52,000 women participate in the population breast cancer screening program in the hospital where the study was conducted, around 4,150 participants according to the NLST criteria and 6,300 participants according to the NELSON criteria would be eligible if lung cancer screening were implemented.
Consumption pattern and proportion of women at high risk of developing lung cancer according to the NLST and NELSON criteria among survey respondents (Hospitalet de Llobregat, 2016).
| Smokers | Former smokers | |
|---|---|---|
| n = 299 | n = 318 | |
| Age at starting smoking (years) | 20.8 (19.9–21.6) | 19.7 (19.0–20.4) |
| Time since cessation (years) | – | 16.1 (14.8–17.5) |
| Cigarettes/day | 11.9 (11.1–12.7) | 15.1 (13.7–16.5) |
| Time smoking (years) | 37.6 (36.8–38.5) | 24.1 (22.7–25.5) |
| Lifetime consumption (pack-years) | 22.7 (21.0–24.3) | 20.4 (18.0–22.7) |
| Risk of lung cancer | ||
| NLST criteria | 23.4% (18.6–28.2) | 18.2% (14.2–22.5) |
| NELSON criteria | 42.8% (37.2–48.4) | 20.8% (16.3–25.2) |
Mean (95% CI) for quantitative variables, proportion (95% CI) for variable “risk of lung cancer”.
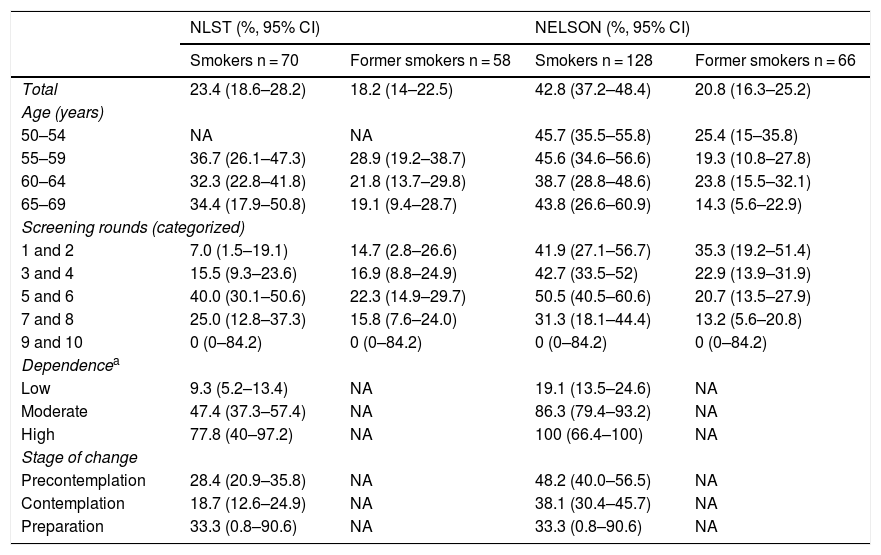
Table 2 shows the proportions of smokers and former smokers at high risk stratified by the variables age, round of participation, brief Fagerström test, and stage of change. The proportions of smokers at high risk are similar among the different age groups, with the highest prevalence occurring among the group aged 55−59 years according to the NLST criteria, and 50–54 years according to the NELSON criteria. A statistically significant upward trend was observed in the proportion of women with high risk as nicotine dependence increases according to the brief Fagerström test. In addition, according to the NELSON criteria, there was a downward, but not statistically significant, trend in the proportion of women who present high risk as the stage of change shifts toward the preparation stage (Table 2).
Proportion (95% CI) of women smokers and former smokers with high risk according to NLST and NELSON criteria stratified by the variables age, current screening round, nicotine dependence, and stage of change (Hospitalet de Llobregat, 2016).
| NLST (%, 95% CI) | NELSON (%, 95% CI) | |||
|---|---|---|---|---|
| Smokers n = 70 | Former smokers n = 58 | Smokers n = 128 | Former smokers n = 66 | |
| Total | 23.4 (18.6–28.2) | 18.2 (14–22.5) | 42.8 (37.2–48.4) | 20.8 (16.3–25.2) |
| Age (years) | ||||
| 50–54 | NA | NA | 45.7 (35.5–55.8) | 25.4 (15–35.8) |
| 55–59 | 36.7 (26.1–47.3) | 28.9 (19.2–38.7) | 45.6 (34.6–56.6) | 19.3 (10.8–27.8) |
| 60–64 | 32.3 (22.8–41.8) | 21.8 (13.7–29.8) | 38.7 (28.8–48.6) | 23.8 (15.5–32.1) |
| 65–69 | 34.4 (17.9–50.8) | 19.1 (9.4–28.7) | 43.8 (26.6–60.9) | 14.3 (5.6–22.9) |
| Screening rounds (categorized) | ||||
| 1 and 2 | 7.0 (1.5–19.1) | 14.7 (2.8–26.6) | 41.9 (27.1–56.7) | 35.3 (19.2–51.4) |
| 3 and 4 | 15.5 (9.3–23.6) | 16.9 (8.8–24.9) | 42.7 (33.5–52) | 22.9 (13.9–31.9) |
| 5 and 6 | 40.0 (30.1–50.6) | 22.3 (14.9–29.7) | 50.5 (40.5–60.6) | 20.7 (13.5–27.9) |
| 7 and 8 | 25.0 (12.8–37.3) | 15.8 (7.6–24.0) | 31.3 (18.1–44.4) | 13.2 (5.6–20.8) |
| 9 and 10 | 0 (0–84.2) | 0 (0–84.2) | 0 (0–84.2) | 0 (0–84.2) |
| Dependencea | ||||
| Low | 9.3 (5.2–13.4) | NA | 19.1 (13.5–24.6) | NA |
| Moderate | 47.4 (37.3–57.4) | NA | 86.3 (79.4–93.2) | NA |
| High | 77.8 (40–97.2) | NA | 100 (66.4–100) | NA |
| Stage of change | ||||
| Precontemplation | 28.4 (20.9–35.8) | NA | 48.2 (40.0–56.5) | NA |
| Contemplation | 18.7 (12.6–24.9) | NA | 38.1 (30.4–45.7) | NA |
| Preparation | 33.3 (0.8–90.6) | NA | 33.3 (0.8–90.6) | NA |
NA: Not applicable.
According to the NLST and NELSON criteria respectively, around 20% and 40% of smokers who participated in this population breast cancer screening program were at high risk of developing lung cancer. Moreover, 20% of former smokers were at high risk according to these same criteria. According to our estimates, of the 80,000 women invited for breast cancer screening in our hospital, almost 9,300 according to the NLST criteria and more than 13,000 according to the NELSON criteria would be candidates for a hypothetical lung cancer screening program.
The results according to the NLST criteria are similar to those described in a Spanish study based on National Health Survey of Spain (ENSE) data for 2011–2012, which estimated the percentage of women with a high risk of lung cancer among women who had participated in a breast cancer screening program in the past 3 years.26
On a European level, among patients in the age range for breast cancer screening, about 30% of smokers and 8% of former smokers according to the NLST criteria and 70% of smokers and 14% of former smokers according to the NELSON criteria were estimated to have a high risk of developing lung cancer.27 The smaller proportions of smokers and the greater proportions of former smokers with high risk observed in our study compared with EU figures are consistent with differences between both populations in the cumulative average lifetime consumption of tobacco. Mean estimated consumption among smokers in the EU was 27.6 pack-years, while in our series, it was 22.7 pack-years. The cumulative consumption among former smokers in the EU was estimated at 14.1 pack-years, while in our series, it was 20.4 pack-years. These differences could be due to the lower educational level of our series compared to average EU standard,28 and the known inverse relationship between educational level and tobacco consumption.29
Our results indicate that if consumption remains constant, the largest increases in the proportion of women entering the high risk category would occur, according to the NLST criteria, in women in the 50–54 and 55−59 year age groups. This estimated transition to a high-risk status in subsequent rounds, which mainly occurs when using the NLST criteria, highlights differences in the restrictiveness of the NLST and NELSON criteria, the second set being much more lax both in terms of age and cumulative consumption in pack-years, accelerating the shift to high-risk status determined according to these criteria.
In our study, however, none of the former smokers will become high-risk in the next few years (data not shown). This situation is consistent with the dichotomization of the risk associated with the use of the NLST and NELSON criteria, because once former smokers have quit smoking, they can only pass into the high risk situation when they meet the age criterion, since their cumulative consumption does not change. In the case of breast cancer screening, all women in the population are over the age of 50, so they have all (NELSON) or nearly all (NSLT) met the age criterion already. In this regard, we believe that former smokers at high risk could benefit from participation in lung cancer screening, especially in view of the preliminary results of the NELSON study in which a gender trend in favor of women was observed, with a higher reduction in lung cancer mortality in women than in men: around 26% (p = 0.003) in men and 39% (p = 0.0543) in women after 10 years of follow-up.21 Moreover, as mentioned in previous discussions on the possible implementation of this type of screening, offering this opportunity to people who have quit smoking would also be a matter of medical ethics.30
We found that the highest proportion of high-risk subjects were among those who are highly dependent on nicotine, as measured by the brief Fagerström test. This result is in line with the findings of another study that retrospectively showed a close correlation between the Fagerström score in 171 subjects and prior exposure to nicotine (as an indicator of lung cancer risk).31 This suggests that the brief Fagerström test could also be used as an indicator of a high risk of lung cancer.
Our study has certain limitations that warrant mention. Firstly, the NLST and NELSON criteria, which incorporate only the variables age and cumulative consumption of tobacco, are less accurate in determining the high-risk population than risk prediction models that include other individual factors, such as exposure to carcinogenic substances (radon, asbestos), genetic factors, underlying diseases, etc.32 In particular, the NLST and NELSON criteria were less sensitive than the predictive models,33 mainly because of the absence of the aforementioned variables that are included in the prediction models, and which account for a substantial part of lung cancer risk. In this respect, our results probably underestimate the proportion of women who are high-risk, so a significant number of lung cancers could go undetected. This situation has already been observed in certain lung cancer registries in different countries, in which a limited proportion of lung cancer patients met the NLST criteria.34 Furthermore, when calculating the cumulative consumption of tobacco, current consumption was taken as a constant from the start of consumption, an approach that might have slightly modified, and probably overstated, the real proportion of women at risk. We should also mention the possible effect on our results of the bias inherent to questionnaire-based studies, such as recall bias or the healthy volunteer effect. This may have led us to underestimate the proportion of the at-risk population.
In conclusion, around 20%–40% of smokers and around 20% of former smokers who participated in this study had a high risk of developing lung cancer, and as such would be candidates for participation in a possible lung cancer screening program. Given the high level of participation in our study, we believe that smoking cessation should be promoted within breast cancer screening programs, taking advantage of the situation as a teachable moment, even if lung cancer screening programs are ultimately introduced in the EU.
Conflict of interestsThe authors state that they have no conflict of interests.
We thank all study participants and the Ministry of Universities and Research of the Government of Catalonia for their support (2017SGR608).
Please cite this article as: González-Marrón A, Martín-Sánchez JC, Garcia-Alemany F, Martínez-Martín E, Matilla-Santander N, Cartanyà-Hueso À et al. Estimación del riesgo de cáncer de pulmón en mujeres que participan en un programa de cribado poblacional de cáncer de mama. Arch Bronconeumol. 2020;56:277–281.