There is currently no universally accepted definition of asthma−COPD overlap (ACO).
ObjectiveTo compare the prevalence of ACO in patients with asthma or COPD, and to assess their clinical characteristics and the capacity of the different definitions to predict the risk of exacerbation.
MethodProspective observational study with a 12-month follow-up in an asthma cohort and a COPD cohort. Four diagnostic criteria were compared: A) the Spanish 2012 consensus; B) the 2016 international consensus; C) the 2017 consensus between the Spanish COPD guidelines (GesEPOC) and GEMA asthma guidelines; and D) the single criterion of ≥300 eosinophils/μL, proposed by GOLD 2019. The risk of exacerbations was evaluated in each group.
ResultsA total of 345 patients were included, 233 (67.5%) with COPD and 112 (32.5%) with asthma, aged 63 ± 14 years, 70.4% men. Fifteen (4.3%) patients met the criteria for ACO according to the criteria described under A above; 30 (8.7%) with the criteria of B; 118 (34.2%) with the criteria of C; and 97 (28.1%), with the D criterion. The ACO-COPD subtype were older, had worse lung function, and an increased risk of exacerbation compared with the ACO-asthma group. Of all the definitions evaluated, those which distinguished a higher risk of exacerbations were the GesEPOC-GEMA consensus and the GOLD proposal.
ConclusionsThe prevalence of ACO varies enormously depending on the diagnostic criteria used. The ACO population is heterogeneous, and the ACO-COPD subtype is very different from the ACO-asthma subtype. The definitions that include eosinophilia identify ACO patients with a greater risk of exacerbation.
En la actualidad no disponemos de una definición de solapamiento asma-EPOC (ACO) universalmente aceptada.
ObjetivoComparar la prevalencia del ACO en pacientes con asma o EPOC, valorar sus características clínicas y la capacidad predictiva de riesgo de agudización de las diferentes definiciones.
MétodoEstudio prospectivo observacional con seguimiento de 12 meses sobre una cohorte de asma y otra con EPOC. Se comparan 4 criterios diagnósticos: A) el consenso español del 2012; B) el internacional del 2016; C) el consenso entre la guía española de la EPOC (GesEPOC) y la del asma (GEMA) del 2017; y D) el criterio único de ≥300 eosinófilos/μL, propuesto por GOLD 2019. En cada grupo se evaluó el riesgo de agudizaciones.
ResultadosSe incluyeron 345 pacientes, 233 (67,5%) con EPOC y 112 (32,5%) con asma, con una edad de 63 ± 14 años, 70,4% de hombres. Quince (4,3%) pacientes cumplían criterios de ACO con criterios A; 30 (8,7%) con los B; 118 (34,2%) con los C; y 97 (28,1%), con el criterio D. El subtipo ACO-EPOC presentó más edad, peor función pulmonar y mayor riesgo de agudización que los ACO-asma. De todas las definiciones evaluadas, las que presentaron mayor riesgo de agudizaciones fueron la del consenso GesEPOC-GEMA y la propuesta de GOLD.
ConclusionesLa prevalencia de ACO varia enormemente en función del criterio diagnóstico utilizado. La población de ACO es heterogénea, con un subtipo ACO-EPOC muy diferente al ACO-Asma. Las definiciones que incluyen la eosinofília identifican a los ACO de más riesgo de agudización.
Asthma and chronic obstructive pulmonary disease (COPD) are 2 highly prevalent chronic respiratory diseases1 that differ in both their pathogenesis and diagnosis, treatment and prognosis. Nevertheless, it is common in clinical practice to find patients who share similar traits. This condition is known as asthma-COPD overlap (ACO). Studies suggest that ACO patients have more symptoms, worse quality of life, and greater risk of exacerbations than patients with COPD, although their survival is longer.2–5 The response to treatment with inhaled corticosteroids (ICS) in ACO has also been shown to lie halfway between that of COPD and asthma.6,7
The prevalence of ACO in the general population ranges from 1.6% to 4.5%. In patients with COPD, it is between 12.1% and 55.2%, and in patients with asthma, between 13.3% and 61%.7–10 These wide variations reflect the type of population analyzed, the definition of ACO, and in particular, the different criteria used for the identification of ACO. In fact, there is not yet one universally accepted definition of ACO.
In 2012, a consensus document in Spanish11 first proposed certain diagnostic criteria for ACO. Since then, a number of proposals for classification have been made, with varying degrees of acceptance. The most widely accepted are Sin et al.12 and the recent consensus between the Spanish COPD guidelines (GesEPOC) and the Spanish guidelines for the management of asthma (GEMA).13 All these proposals combine largely similar clinical, functional, and/or analytical parameters, but each has small differences that may influence outcomes (Table 1). Taking a different approach, several recent studies found an association between eosinophil count in peripheral blood, risk of worsening, and response to ICS in the prevention of exacerbations.14–17 These findings suggest that eosinophilia might simplify the detection of ACO. The 2019 Global Obstructive Lung Disease Initiative (GOLD) recommends the use of eosinophil counts in peripheral blood in patients at high risk of exacerbations to identify ICS responders.18
ACO diagnostic criteria.
| A | B | C | D |
|---|---|---|---|
| Soler-Cataluña et al.11 | Sin et al.12 | GesEPOC-GEMA consensus13 | Eosinophilic ACO |
| 2012 | 2016 | 2017 | 201918 |
| Major criteria | Major criteria: | - COPD diagnosis (according to GesEPOC) + Current diagnosis of asthma (according to GEMA) | - COPD diagnosis + blood eosinophilia ≥ 300 cells/μl |
| - BD with increase in FEV1 ≥ 15% and ≥400 ml | FEV1/FVC postBD < 0.70 | ||
| - Sputum eosinophilia | - Smoking >10 pack-years | ||
| - Personal history of asthma (before the age of 40) | - History of asthma before the age of 40 or BD > 400 ml | - COPD diagnosis + BD ≥ 15% and 400 ml | |
| Minor criteria: | Minor criteria: | ||
| - Raised total IgE | - History of atopy or allergic rhinitis | ||
| - Personal history of atopy | - BD ≥ 200 ml and 12% | - COPD diagnosis + blood eosinophilia ≥300 cells/μl | |
| - BD with increase in FEV1 ≥12% and ≥200 ml on 2 or more occasions | - Peripheral eosinophilia ≥300 cells/μl | ||
| Two major criteria, or one major and 2 minor criteria are required for the correct diagnosis of this clinical entity in a patient diagnosed with COPD | Three major criteria and at least 1 minor criterion are required | Any of the 3 criteria may be met |
ACO: Asthma-COPD overlap; BD: bronchodilator test; postBD: post-bronchodilation; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; GEMA: Spanish asthma guidelines; GesEPOC: Spanish COPD guidelines; IgE: immunoglobulin E.
In the absence of a gold standard for the diagnosis of ACO, the impact of various classifications must be analyzed in order to propose criteria that best identify this population. The main objective of our study was to compare the prevalence of ACO and ACO-asthma and ACO-COPD subtypes in 2 well-defined patient cohorts, one with asthma and the other with COPD, defined according to the different diagnostic criteria. Secondary objectives were: a) to evaluate if the ACO profile differs in patients with asthma or COPD; and b) to compare the predictive capacity of the risk of exacerbations according to the diagnostic criteria used, the patient cohort, and their response to treatment with ICS.
MethodologyDesignThis was a prospective observational study with a 12-month follow-up in an asthma cohort and a COPD cohort. In both cohorts, 4 diagnostic criteria for ACO were compared: A) those described by Soler-Cataluna et al.11; B) the international consensus of Sin et al.12; C) the Spanish GesEPOC-GEMA consensus13; and D) the GOLD criterion of peripheral eosinophilia ≥300 cells/μl18 (Table 1). Patients were classified into 4 groups on the basis of these criteria: 1) asthma; 2) COPD; 3) ACO from the COPD cohort (ACO-COPD); and 4) ACO from the ACO-asthma cohort (ACO-asthma). The study was carried out in accordance with the Declaration of Helsinki and was approved by the clinical research ethics committee of the Hospital Arnau de Vilanova (Valencia). All patients signed an informed consent form.
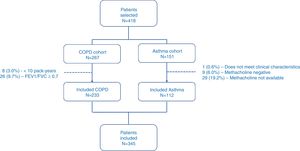
PatientsWe included patients diagnosed with asthma and/or COPD, according to GEMA and GesEPOC diagnostic criteria, respectively19,20 attending pulmonology outpatient clinics. The patients were included in a stable phase, at least after 6 weeks of the resolution of their last exacerbation. Patients who did not sign informed consent and those with a prior diagnosis of primary bronchiectasis, active malignancy, or who were unavailable for subsequent follow-up were excluded from the study.
MeasurementsTo evaluate the diagnostic criteria of ACO, we collected demographic data, smoking history, and clinical characteristics of asthma (symptoms, personal and family history, rhinitis and/or atopy) and performed forced spirometry with bronchodilator testing, according to the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) guidelines.21 At the time of the initial visit, the fraction of exhaled nitric oxide (FeNO) was also evaluated, using the FeNO NiOX® analyzer (Aerocrine, Solna, Sweden), and peripheral eosinophilia and total serum IgE were determined. Cytologic analysis of sputum was not performed.
In all cases, COPD Assessment Test (CAT)22 and the Asthma Control Test (ACT)23 were performed, both at baseline and at the end of the study. We also evaluated the degree of dyspnea using the modified Medical Research Council scale (mMRC)24 and the number and severity of exacerbations in the previous year.
To assess the capacity of each criterion to predict risk of exacerbation, we analyzed time to first exacerbation, time to hospitalization, and exacerbation rate during the year of follow-up. COPD and/or asthma exacerbations were defined according to the accepted definitions of GEMA and GesEPOC.19,20 Finally, the treatment prescribed was recorded paying special attention to the presence or absence of ICS. The prescribing doctor was unaware of the ACO criterion used and was free to use the most appropriate medical treatment according to routine clinical practice.
Statistical analysisAn analysis of variance was used to compare means between different groups, and a Chi-squared test with Bonferroni adjustment was used to compare proportions. The risk of events (exacerbations or hospitalizations) was evaluated with a Cox survival analysis. The Kaplan-Meier method was used to obtain survival curves and the C statistic was calculated to compare the predictive capacity of each of the diagnostic criteria. To calculate the sample size, we estimated that the difference in prevalence of ACO between criteria would be at least a 10%, with a type I error of 5% (alpha = 0.05), single-tailed approach, and minimum required power of 80% (β = 0.20). With these estimates, the sample size calculated for the COPD cohort was 232 patients (for a maximum prevalence of 25% and a minimum of 15%), while the sample size for the asthma cohort was 130 (estimated maximum prevalence of 15% and minimum 5%). The results were defined as statistically significant at p < 0.05. All analyses were performed using the SPSS statistical package version 20.0 (Chicago, Illinois, USA).
ResultsPatientsFrom January to September 2017, a total of 418 patients were selected, of which 73 (17.5%) were excluded for different reasons (Fig. 1). Finally, 345 patients were included in the study, of which 233 (67.5%) had COPD, and 112 (32.5%) had asthma. Table 2 shows the descriptive characteristics of the sample.
Study patient characteristics.
| Total (n = 345) | COPD (n = 233) | Asthma (n = 112) | p | |
|---|---|---|---|---|
| Age (years) | 63 ± 14 | 68 ± 9 | 53 ± 16 | <0.001 |
| Sex | ||||
| Men | 243 (70.4%) | 198 (85.0%) | 45 (40.2%) | <0.001 |
| Smoking | <0.001 | |||
| Never | 70 (20.3%) | 0 (0.0%) | 70 (62.5%) | |
| Former smoker | 176 (51.0%) | 152 (65.2%) | 24 (21.4%) | |
| Active smoker | 99 (28.7%) | 81 (34.8%) | 18 (16.1%) | |
| Pack-years | 52 ± 33 | 58 ± 32 | 18 ± 14 | <0.001 |
| FVC postBD (ml) | 1872 ± 886 | 2997 ± 878 | 3520 ± 1230 | <0.001 |
| FVC postBD (ml) | 68 ± 23 | 83 ± 19 | 95 ± 15 | <0.001 |
| FEV1 postBD (ml) | 3167 ± 1034 | 1529 ± 552 | 2583 ± 1017 | <0.001 |
| FEV1 postBD (%) | 87 ± 19 | 57 ± 18 | 89 ± 18 | <0.001 |
| FEV1/FVC postBD | 58 ± 16 | 51 ± 12 | 73 ± 12 | <0.001 |
| Bronchodilator test (ml) | 158 ± 188 | 116 ± 171 | 244 ± 192 | <0.001 |
| Bronchodilator test (%) | 9 ± 11 | 8 ± 11 | 12 ± 9 | 0.002 |
| Bronchodilator test ≥12% and ≥200 ml | 117 (39.2%) | 42 (18.0%) | 75 (66.9%) | <0.001 |
| Bronchodilator test ≥15% and ≥400 ml | 29 (8.4%) | 13 (5.6%) | 16 (14.3%) | 0.006 |
| FeNO (ppb) | 28 ± 29 | 23 ± 17 | 44 ± 40 | <0.001 |
| FeNO ≥ 50 ppb | 48 (13.9%) | 18 (7.7%) | 30 (26.8%) | <0.001 |
| Peripheral eosinophilia (cells/ml) | 304 ± 335 | 256 ± 189 | 402 ± 508 | 0.004 |
| Peripheral eosinophilia (%) | 4.9 ± 4.7 | 3.1 ± 1.8 | 4.5 ± 3.6 | <0.001 |
| Peripheral eosinophilia ≥300 cells/μl | 156 (45.2%) | 89 (38.2%) | 67 (59.8%) | <0.001 |
| IgE | 232 ± 425 | 227 ± 455 | 240 ± 363 | NS |
| IgE ≥ 100 kUA/l | 159 (46.1%) | 105 (45.1%) | 54 (48.2%) | <0.001 |
PostBD: post-bronchodilation; COPD: chronic obstructive pulmonary disease; FeNO: fraction of exhaled nitric oxide; FEV1: force expiratory volume in the first second; FVC: forced vital capacity; IgE: immunoglobulin E; NS: not significant.
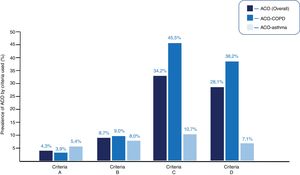
Fifteen (4.3%) patients met ACO criteria for criteria A; 30 (8.7%) for B; 118 (34.2%) for C; and 97 (28.1%) for D (p < 0.001, for each comparison between criteria). Appendix B Table 1s (supplementary material) specifies the degree of compliance with each of the required criteria.
In the COPD cohort, 9 (3.9%), 21 (9.0%), 106 (45.5%), and 89 (38.2%) had ACO-COPD, according to criteria A, B, C, and D, respectively (p < 0.001). In the asthma cohort, 6 (5.4%), 9 (8.0%), 12 (10.7%) and 8 (7.1%) had ACO-asthma, according to criteria A, B, C, and D, respectively (p < 0.001). Fig. 2 shows the prevalence of overall ACO, ACO-COPD, and ACO-asthma, according to each set of criteria.
Different clinical profiles in asthma-COPD overlapAppendix B Tables 2 As-Ds (supplementary material) show the different characteristics of each group, according to the different criteria used. In general, patients with ACO had an intermediate profile between asthma and COPD. No differences were observed in ACT and CAT scores between the groups for any of the 4 definitions. The proportion of patients treated with ICS was similar in the 4 groups: long-acting beta2-adrenergic agonists (LABA)/ICS were more frequently used by asthma and ACO-asthma patients, and the triple combination of long-acting antimuscarinic antagonists (LAMA)/LABA/ICS was more frequent among COPD and ACO-COPD patients.
Asthma-COPD overlap subtypesPatients with ACO-COPD were older, a higher proportion were males, and they had worse lung function, more symptoms and more exacerbations in the last 12 months than ACO-asthma patients.
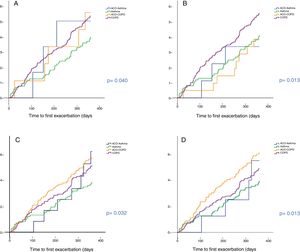
Predictive capacity of risk of the different definitionsTime to first exacerbationFig. 3 shows the time to first exacerbation according to the 4 definitions of ACO evaluated. Patients with ACO-COPD showed the greatest risk of exacerbation when criteria C and D were used. This risk was greater than in cases with asthma (p < 0.05). No statistically significant differences were found between ACO-COPD and COPD, or between ACO-asthma and ACO-COPD for criteria C. When criteria D were used, an increased risk of exacerbation was found for the ACO-COPD group compared to the COPD group, although the difference was not significant (p = 0.094). The C statistic was 0.64 for criteria C and 0.62 for criteria D. The cumulative probability of presenting an exacerbation was 57% and 61% in ACO-COPD patients compared with 51% and 49% in COPD patients for criteria C (p = NS) and D (p = 0.094), respectively.
Patients with ACO-asthma behaved similarly to patients with asthma, with no significant differences between them for any of the 4 definitions.
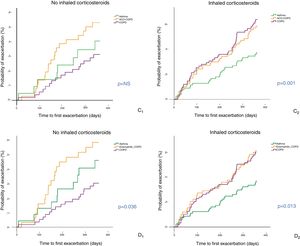
Analysis according to treatment with inhaled corticosteroidsFig. 4 shows the Kaplan–Meier curves when criteria C and D were used, according to use or non-use of ICS. In the cohort that received no ICS, patients with ACO-COPD showed increased risk of exacerbation, with significant differences compared with COPD patients (p < 0.01 in D and p = 0.059 in C). Among those who received ICS (either in the form of LABA/ICS or as triple combination [LAMA/LABA/ICS]), the risk of exacerbation was lower only for asthma patients (p < 0.01).
Time to first exacerbation of patients with COPD, asthma and ACO-COPD, according to use of inhaled corticosteroids (ICS) Cases with OAC-asthma are excluded from the figure due to their low prevalence among subgroups. C: criteria of Plaza et al.13 (C1: patients not receiving ICS; (C2: patients receiving ICS); D: GOLD 201918 (D1: patients not receiving ICS; (D2: patients receiving ICS).
In the COPD cohort, patients treated with ICS had a higher previous history of exacerbations in the last 12 months (1.3+-2.1 vs. 1.3 ± 0.6 ± , p < 0.001), and an overall tendency towards a higher risk of exacerbation during the year of follow-up (HR: 1.35; p = 0.063). Despite this, the risk of exacerbation between COPD and ACO-COPD was similar.
Multivariate analysisAfter performing multivariate analysis, adjusting the model for age, post-bronchodilator FEV1%, exacerbations in the last year, and initial treatment with ICS, none of the ACO definitions proved to be an independent predictor of exacerbations. Table 3 shows the adjusted risk of criteria C and D.
Influence of the diagnostic criteria C and D on the risk of exacerbations. Multivariate analysis.
| HR: | 95% CI HR | p | |
|---|---|---|---|
| Age | 1.021 | 1.007–1.036 | 0.004 |
| FEV1 postBD (%) | 0.989 | 0.980–0.998 | 0.019 |
| No. exacerbations in previous year | 1.160 | 1.090–1.235 | <0.001 |
| Criterion C | NS | ||
| Asthma | – | – | – |
| ACO-asthma | 1.209 | 0.501–2.915 | NS |
| ACO-COPD | 1.094 | 0.641–1.869 | NS |
| COPD | 0.892 | 0.588–1.588 | NS |
| Age | 1.021 | 1.006–1.036 | 0.005 |
| FEV1 postBD (%) | 0.989 | 0.980–0.998 | 0.013 |
| No. exacerbations in previous year | 1.160 | 1.091–1.234 | <0.001 |
| Criterion D | NS | ||
| Asthma | – | – | – |
| ACO-asthma | 0.930 | 0.286–3.030 | NS |
| ACO-COPD | 1.126 | 0.679–1.866 | NS |
| COPD | 0.879 | 0.543–1.425 | NS |
ACO: Asthma-COPD overlap; COPD: chronic obstructive pulmonary disease; CI: confidence interval; FEV1: forced expiratory volume in the first second; HR: hazard ratio; NS: not significant.
Table 4 shows the average rate of exacerbations during the year of follow-up for each of the study groups, according to the different criteria evaluated. The main difference between the 4 criteria lies in the annual rate of exacerbations in groups C and D. There were no statistically significant differences between the rate of hospitalizations.
Annual rate of exacerbations Comparison between the 4 criteria.
| Asthma | ACO-asthma | ACO-COPD | COPD | p | |
|---|---|---|---|---|---|
| Number of exacerbations/year | |||||
| Criteria A | 0.63 ± 1.01 | 0.67 ± 0.81 | 0.89 ± 0.93 | 1.11 ± 1.56 | 0.035 |
| Criteria B | 0.65 ± 1.02 | 0.44 ± 0.72 | 0.52 ± 0.86 | 1.16 ± 1.59 | 0.006 |
| Criteria C | 0.63 ± 1.04 | 0.67 ± 0.65 | 1.13 ± 1.47 | 1.08 ± 1.60 | 0.037 |
| Criteria D | 0.63 ± 1.02 | 0.62 ± 0.74 | 1.20 ± 1.48 | 1.03 ± 1.58 | 0.027 |
| Number of hospitalizations/year | |||||
| Criteria A | 0.03 ± 1.67 | 0.17 ± 0.41 | 0.00 ± 0.00 | 0.12 ± 0.47 | NS |
| Criteria B | 0.03 ± 1.70 | 0.11 ± 0.31 | 0.00 ± 0.00 | 0.13 ± 0.48 | NS |
| Criteria C | 0.02 ± 0.14 | 0.17 ± 0.39 | 0.07 ± 0.25 | 0.16 ± 0.58 | 0.056 |
| Criteria D | 0.02 ± 0.14 | 0.25 ± 0.46 | 0.07 ± 0.25 | 0.15 ± 0.58 | 0.053 |
ACO: Asthma-COPD overlap; COPD: chronic obstructive pulmonary disease; NS: not significant.
Our study compares different proposals for defining ACO in a cohort of patients with COPD and in another cohort with asthma, specifically evaluating overall prevalence and prevalence by ACO subtypes, their risk of exacerbation, and their response to treatment with ICS. The main findings of the study were as follows: 1) the proportion of patients with ACO varied substantially, between 4.3% and 34.2% according to the definition used; 2) ACO-COPD patients presented a completely different profile from those with ACO-asthma; 3) the classifications that identified ACO-COPD patients with an increased risk of exacerbations were the Spanish GesEPOC-GEMA consensus and the criterion of eosinophilic ACO; unexpectedly, however, this increased risk disappeared after adjusting for confounding variables; and 4) among patients not treated with ICS, the risk of exacerbation was significantly higher for ACO-COPD than for COPD. This risk was the same among patients who used ICS.
Prevalence of asthma-COPD overlapThe prevalence of ACO described in the literature varies greatly between studies. This variation has been attributed to differences in the study populations, to the use of different criteria to define asthma and/or COPD, and, in particular, to differences in ACO criteria.13 In our study, the different criteria were applied to the same population, and COPD and asthma were defined scrupulously according to the GEMA and GesEPOC guideline,19,20 so the differences observed can only be explained by the criteria used. The most restrictive of all the criteria are those of the 2012 Spanish consensus document,11 which showed a prevalence of ACO of only 4.5%. Calle et al.,25 in a cross-sectional study of 647 patients with COPD, found a prevalence of 6.5%, similar to our own. However, prevalence rises to 15% when these criteria are modified slightly, and peripheral blood eosinophilia >5% is included as a minor criterion and only one major criterion or 2 minor criteria are required to define ACO.3 Eosinophilia in sputum was not determined in either of these 2 studies3,25 or in ours, so we cannot rule out an underestimation of the prevalence of ACO. However, the cytologic analysis of sputum is, unfortunately, a technique used in very few centers, so we believe that the cited prevalences are closer to what most clinicians might encounter in their clinical practice.
The proposal of Sin et al.12 has been validated in a recent Korean study.26 Applying these criteria, the authors found a prevalence of ACO of only 1.9%, which, moreover, did not affect the annual risk of exacerbation. In our series, the prevalence of ACO was 8.7% and, as is the case with the Korean study, the ability to predict risk was no different for patients with ACO.
Compared to these results, the most recent proposals give a substantially higher proportion of ACO. The prevalence of ACO in our study was 34.2% using the criteria proposed by the GesEPOC-GEM consensus,13 and 28.1% using the sole criterion of eosinophilia ≥300 cells/μl. The proportion of cases with ≥300 eosinophils/μl was 45.2% (59.8% in the asthma cohort and 38.2% in the COPD cohort), which undoubtedly contributes to a substantial increase in the prevalence of ACO according to criteria C and D. Although these figures are high, other series present similar results. Pérez de Llano et al.27 found that 29.8% of the 292 patients included in a Spanish multicenter study met the criteria for ACO according to the SEPAR consensus document.13 More recently, Toledo-Pons et al.,28 in a retrospective analysis of 603 patients in the Majorica cohort in the Balearic Islands, also reported similar results, with a prevalence of ACO of 27.4% when using these same criteria.13 Approximately half of the cases were diagnosed exclusively on the basis of ≥300/μl eosinophils in peripheral blood.
Asthma-COPD overlap subtypesRecent data suggest that patients with ACO are not a homogeneous group, and clinical and biological differences exist between asthmatics who smoke and COPD patients with eosinophilia.28–30 Our data reinforce this hypothesis because, regardless of the diagnostic criteria used, patients with ACO-COPD showed a different clinical profile with a greater proportion of men, older age, worse lung function, more symptoms, and also a greater risk of exacerbation than ACO-asthma patients. One of the points of current debate revolves around the importance of isolated peripheral blood eosinophilia in patients no other ACO criteria. According to Song et al.,31 eosinophil levels in patients without ACO was not associated with changes in FEV1 or with changes in health-related quality of life or the frequency of exacerbations, so the significance of this type of isolated peripheral eosinophilia without other accompanying features of ACO is still to be determined.
Future risk of exacerbationsOf the 4 ACO definitions evaluated, those which identified ACO patients with an increased risk of exacerbations were criteria C and D. Both proposals are relatively similar, in that they have a common criterion: the presence of peripheral eosinophilia. Different studies have associated eosinophil counts with an increased risk of moderate-severe exacerbations.32–35 However, none of these studies adjusted the risk of exacerbation for other confounding variables. Surprisingly, after adjusting for age, FEV1, exacerbations in the preceding year, and initial treatment with ICS, we found that the risk of exacerbation attributable to ACO and/or to peripheral blood eosinophilia disappeared in the multivariate analysis. However, this was an observational study, so we cannot rule out the possibility of interaction with other confounding variables. Several studies have shown that ACO and/or peripheral blood eosinophilia is associated with better prognosis,3,9,36 although no adjustments were made for severity.
Response to treatment with inhaled corticosteroidsIn the absence of more significant differences in clinical outcomes, the only reason to continue to categorize ACO as a clinical phenotype is because patients respond differently to treatment. Some studies suggest that patients with ACO respond better to ICS, particularly those with peripheral eosinophilia.14–16,37–39 As this was an observational study, we cannot draw any direct conclusions on the role of ICS in our series. In fact, patients who received anti-inflammatory treatment were in general more severe and had a greater proportion of prior exacerbations. However, when the risk of exacerbation among the cases that did not receive ICS was examined, ACO-COPD patients showed a higher risk of exacerbation than patients with COPD. This difference in risk disappeared among patients treated with ICS, indirectly suggesting a beneficial effect.
LimitationsOur study is carried out in a single center, so we cannot rule out regional factors that might explain the high peripheral blood eosinophilia. However, as has already been discussed, various Spanish studies presented similar data.27,28,36 The follow-up was 1 year, so we were unable to properly assess survival. Finally, the design of the study was observational, so the potential benefit of ICS treatment in ACO must be judged with caution.
ConclusionsThe prevalence of ACO varies widely, depending on the diagnostic criteria used. The oldest criteria are very restrictive and do not correlate with the risk of exacerbations, while the more recent GesEPOC-GEM proposal or the eosinophil count are more inclusive. Regardless of the proposal used, we confirmed that the ACO population is heterogeneous, with two highly differentiated subtypes: ACO-asthma and ACO-COPD.
Conflict of interestsJuan José Soler-Cataluña has received speaker fees from AstraZeneca, Boehringer Ingelheim, Bial, Ferrer, Laboratorio Esteve, Menarini, Mundipharma, Novartis, Rovi and TEVA; consultancy fees from AirLiquide, AstraZeneca, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, Laboratorios Esteve, Mundipharma and Novartis, and research grants from GlaxoSmithKline and Boehringer Ingelheim.
Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Zambon, CSL Behring, Grifols and Novartis; consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Ferrer, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Laboratories Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, pH Pharma, Novartis, and Grifols, and research grants from GlaxoSmithKline and Grifols.
Fernando José Sánchez-Toril Lopez has received speaker fees from Almirall, AstraZeneca, Boehringer Ingelheim, Bial, Ferrer, GSK, Laboratorio Esteve, Menarini, Mundipharma, Novartis and TEVA, and honoraria for consultancy from AstraZeneca and TEVA.
Laura Novella, Cristina Soler, María Luisa Nieto, and Violet Esteban declare that they have no conflict of interest.
Please cite this article as: Soler-Cataluña JJ, Novella L, Soler C, Nieto ML, Esteban V, Sánchez-Toril F, et al. Características clínicas y riesgo de agudizaciones asociados con diferentes criterios diagnósticos del solapamiento asma-EPOC. Arch Bronconeumol. 2020;56:282–290.