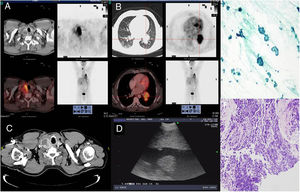
We report the case of a 55-year-old male patient, an active smoker with a 60 pack-year history and previous intestinal polyposis, referred from another center due to the finding on routine chest radiograph of an image suggestive of lung cancer. Chest computed tomography (CT) showed a 57-mm left bilobar hilar lesion and a 40-mm right thyroid nodule. 18Fluorodeoxyglucose positron emission tomography (18F-FDG PET/CT) indicated active metabolism in both, with no mediastinal or extrathoracic involvement, suggesting a diagnosis of T3N0M0 lung cancer, and a hypermetabolic right thyroid nodule in the context of a nodular goiter which could correspond to a tumor or overactive nodule. Thyroid hormone levels requested for analysis of the thyroid nodule were normal. Fiberoptic bronchoscopy was performed, which identified an endobronchial tumor located in the left lower lobe bronchus. Biopsies were positive for small cell lung cancer (SCLC). As this was a patient with suspected lung cancer requiring treatment with radical intent, endobronchial ultrasound (EBUS) was performed simultaneously to complete the staging, using an Olympus BF-UC 180F ultrasonic bronchoscope (Olympus, Tokyo, Japan); no lymph nodes with ultrasound signs of malignancy were identified. Extrinsic compression was also observed in the upper third of the trachea due to the right nodular thyroid lesion, with hypoechoic areas inside. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA)1 of the thyroid nodule was therefore carried out, revealing Hürthle cell follicular carcinoma and thus ruling out a pulmonary origin. Papanicolaou staining was used, as the samples were alcohol-fixed. In the case of the thyroid specimens, samples were air-dried for subsequent Giemsa staining for optimal evaluation. Given these results, it was decided to prioritize treatment of the SCLC, which was staged as T3N0M0 (Stage IIB); a chemotherapy regimen (cisplatin-etoposide) was therefore initiated with concomitant radiotherapy (total dose 64Gy). Meanwhile, the patient continued to have check-ups in endocrinology and, depending on the response of the lung cancer to treatment, hemithyroidectomy would be considered (Fig. 1).
Images A and B, corresponding to axial slices obtained in the 18F-FDG PET/CT study: (A) The images show pathological heterogeneous uptake of FDG, which is mapped onto the right thyroid nodule found in the CT scan. (B) Solid lesion with high FDG uptake in the left hilum with signs of lobular invasion. (C) Axial chest CT at cervical level, showing an increase in the nodular volume of the right thyroid lobe. (D) EBUS image of the right thyroid lobe. (E) TBNA of thyroid: uniform cell population of Hürthle cells distributed in a discohesive manner or in follicles, suggestive of Hürthle cell follicular carcinoma (Papanicolaou staining 40×). (F) Bronchial biopsy: small cell lung cancer (hematoxylin–eosin staining 20×).
Despite the high negative predictive value of 18F-FDG PET/CT—about 95% in mediastinal staging of lung cancer—histopathological confirmation using techniques such as EBUS-TBNA is necessary.1 The use of 18F-FDG PET/CT has highlighted a growing prevalence of thyroid lesions with increased uptake: 47% correspond to malignant disease, either primary or metastatic, mostly lung cancer, breast cancer or lymphoma.2,3 It is important to note that 18F-FDG uptake in the glandular tissue of the thyroid does not differentiate between benign or malignant lesions, so it is advisable to rule out a concomitant neoproliferative process.4.5
EBUS-TBNA is currently the most common procedure for mediastinal staging of non-SCLC, with sensitivity and specificity above 90% and well established safety and effectiveness.6 Sampling of all known mediastinal lesions in these patients confirms disease spread and determines the therapeutic strategy.7 When there is an incidental finding of thyroid lesions by 18F-FDG PET/CT, diagnostic confirmation is also recommended.8 In the thyroid, increased focal uptake is more suggestive of malignancy than diffuse uptake, so its study and follow-up are essential.5 Most thyroid nodules detected in patients with lung cancer are benign. Nevertheless, there are cases of thyroid metastases in these patients, which is why cytological confirmation is essential.9,10
So far, the technique used for sampling these lesions is ultrasound-guided fine needle aspiration biopsy (US-FNAB), or surgery if lesions are inaccessible. However, cases have been described in recent years where EBUS-TBNA has become a good alternative for minimally-invasive histopathological diagnosis of those lesions that are inaccessible or in patients with high surgical risk. The results are promising, and in the future, EBUS-TBNA could even become the diagnostic technique of choice.11–14
Please cite this article as: Bellido-Calduch S, Martín-Ontiyuelo C, Pijuan L, Puig de Dou J, Suárez-Piñera M, Curull V, et al. Diagnóstico endoscópico de carcinoma pulmonar de célula pequeña y neoplasia folicular de tiroides. Arch Bronconeumol. 2020;56:328–329.