Persistent air leak is frustrating for both patients and physicians, above all leaks with a high risk of surgery. Insertion of endobronchial valves could be an alternative to surgery. The aim of this study is to describe our experience in these valves and analyze their efficacy in a series of patients with persistent air leaks.
Materials and methodsThe valves are inserted by means of flexible bronchoscopy under conscious sedation and local anesthesia. A preliminary bronchoscopy identifies the air leak by bronchial occlusion using a balloon catheter. A successful outcome is defined as complete disappearance of the leak following removal of the chest drain, without the need for further surgery.
ResultsFrom November 2010 to December 2013, 8 patients with persistent air leaks were treated with endobronchial valves. The number of valves used ranged from 1 to 4 (median 2), with a median duration of air leak prior to placement of 15.5 days. There were no complications and the resolution of the leak was complete in 6 of 8 patients (75%). The median duration of drainage after insertion of the valves was 13 days and the median time to removal of 52.5 days.
ConclusionsInsertion of endobronchial valves is a safe and effective method for treating persistent air leaks, and a valid alternative to surgery.
La fuga aérea prolongada es motivo de frustración entre médicos y pacientes, sobre todo para aquellos con alto riesgo quirúrgico. El uso de válvulas endobronquiales podría ser una alternativa al tratamiento quirúrgico. El objetivo de este trabajo es mostrar nuestra experiencia con el uso de las mismas y analizar su eficacia en una serie de casos tratados por fuga aérea persistente.
Material y métodosLa colocación de las válvulas se realiza mediante broncoscopia flexible, bajo sedación consciente y anestesia local. La fuga aérea se identifica, en un primer paso, mediante la oclusión del bronquio con un catéter-balón durante una broncoscopia. El éxito del procedimiento se define como la desaparición completa de la misma, con la retirada del drenaje torácico sin necesidad de otros procedimientos posteriores.
ResultadosDe noviembre de 2010 a diciembre de 2013 se han tratado 8 pacientes por fuga aérea persistente con válvulas endobronquiales. El número de válvulas utilizadas osciló entre 1 y 4 (mediana de 2), con una mediana de duración de la fuga aérea previa a su colocación de 15,5días. No hubo complicaciones, y la resolución de la fuga fue completa en 6 de los 8 pacientes (75%). La mediana de duración del drenaje después de la inserción de las válvulas fue de 13días y la mediana del tiempo transcurrido hasta su extracción, de 52,5días.
ConclusionesEl uso de válvulas endobronquiales es un método eficaz y seguro para el tratamiento endobronquial de la fuga aérea prolongada y una alternativa válida a la cirugía.
Persistent air leak (PAL) is caused by an abnormal communication between the alveolar space and the pleural space lasting more than 5–7 days.1
It occurs as a complication in up to 15% of thoracotomies, in spontaneous pneumothorax in patients with underlying lung disease, chest injury, as a complication of radiofrequency ablation of lung tumors, for treatment-related reasons (thoracocentesis, pleural biopsy, transthoracic aspirations) or as a complication of empyema or necrotizing pneumonias.2 It generally develops in patients with chronic lung diseases and/or multiple pathologies, and is associated with long hospital stays, more intensive care unit (ICU) admissions, prolonged use of chest tube drainage, with the resulting increased risk of infection, greater morbidity, and risk of death, all of which are reflected in high healthcare costs.3,4
PAL has traditionally been managed with surgical intervention, however, due to the above-mentioned comorbidities, some patients are inoperable. For this reason, minimally invasive endoscopic techniques have been developed in recent years, including one-way endobronchial valves (EBV). These devices offer the best efficacy and safety available to date.5
EBV were initially designed for the endoscopic treatment of loss of volume in emphysema, and were later introduced for the treatment of PAL. The device works in a similar way to the Heimlich valve, preventing airflow toward the pulmonary areas distal to the valve, while permitting drainage of mucus and air in the proximal direction.6,7 In some cases, collapse of the affected segment and expansion of the normal adjacent lung segment is achieved,8 thus contributing to the reduction or resolution of the leakage defect and subsequent healing of the lung parenchyma.9
We report a series of 8 patients with persistent airflow treated with endobronchial valves, and compare our experience with the results from other series published to date.
Materials and MethodsPopulationA prospective study was conducted in a series of 10 patients who were evaluated for endobronchial valve placement between October 2010 and November 2013 due to persistent air leak after conservative treatment with a chest tube. Patients referred for evaluation were either unsuitable for surgery due to significant comorbidities or subjects whose previous surgery was not effective for controlling PAL.
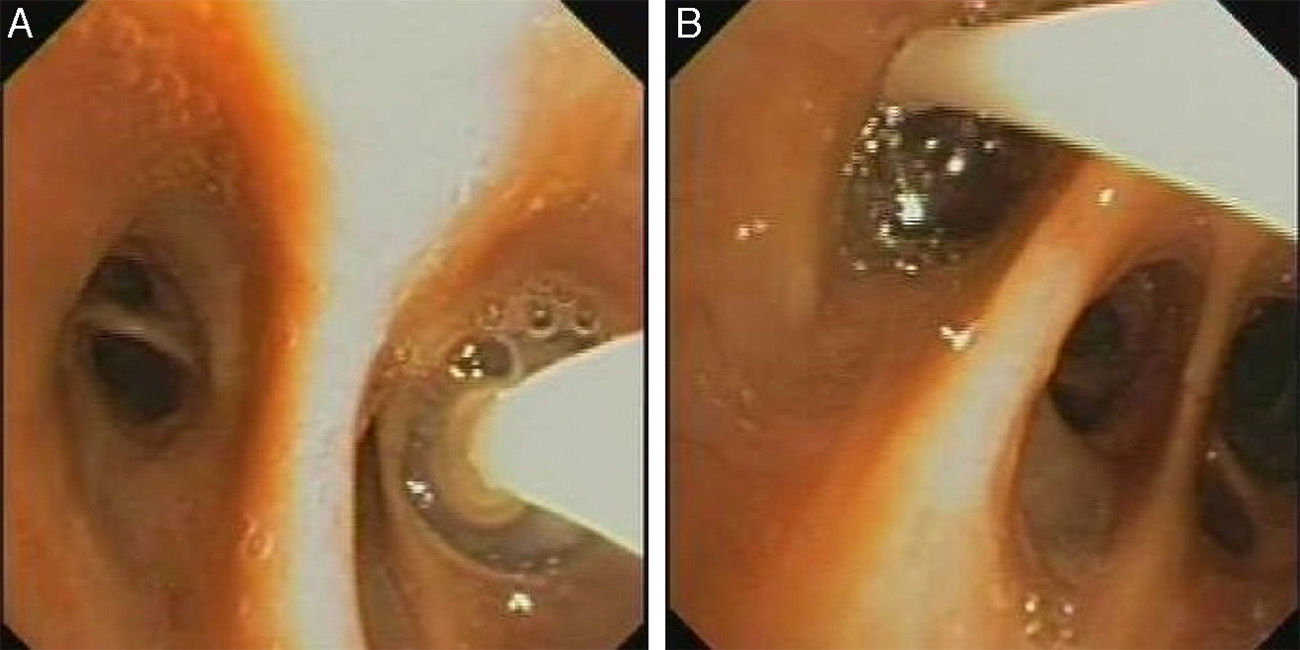
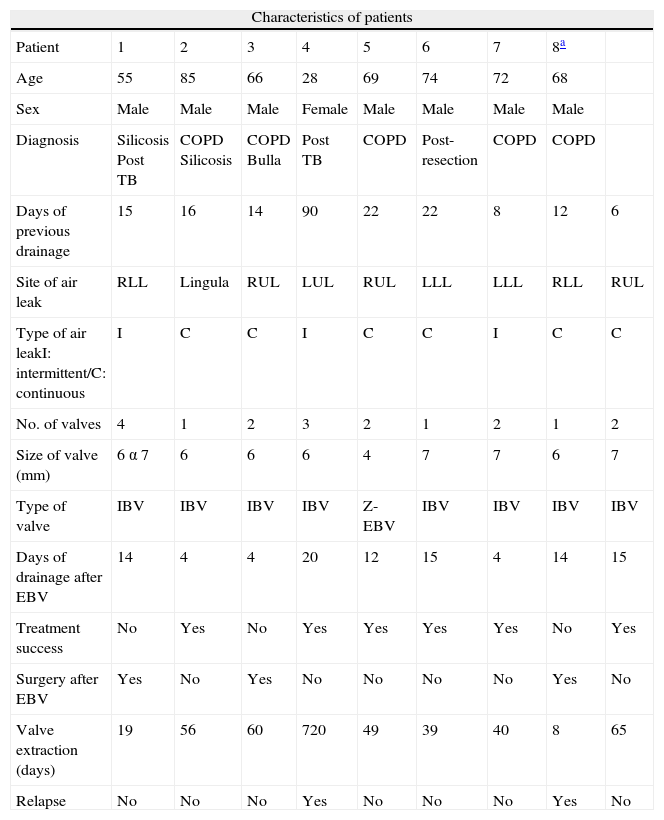
ProcedureValve placement is performed using flexible bronchoscopy, under conscious sedation and local anesthesia. The first step in EBV placement technique is to locate the anatomical origin of the air leak, which is identified using balloon occlusion during flexible bronchoscopy (Fig. 1A and B).
Locating the LeakThe various segments or subsegments are sequentially occluded with a Fogarty balloon catheter, moving proximally to distally. First, the main bronchus is occluded to determine if the air leak stops. If so, blockage of the upper lobe bronchus is sustained for 1–3min while the water seal is checked for bubbling. During the occlusion procedure, it is advisable to wait several respiratory cycles (5 breaths) to determine the effect of the occlusion on the air leak. When the bronchus responsible for the leak is occluded, bubbling will reduce or even disappear.10 If no change occurs in the air leak, the lower lobe bronchus should be isolated and, in the right lung, the middle lobe will be simultaneously occluded. When the target lobe has been identified, the next step is to test each separate segment. This systematic analysis of the bronchial tree means that the leak can usually be located, even when more than one segment or more than one lobe are involved.11
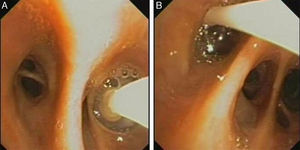
Valve PlacementOnce the source of the fistula has been identified, the next step is to determine the size of the bronchus to select the most suitable valve diameter. Two types are commercially available. One, the IBV® valve (Olympus), is constructed with a nitinol frame with an umbrella-shaped polymer frame; a calibrated balloon is inflated inside the bronchus to determine the correct diameter needed for the valve. The other, the Zephyr® EBV (Pulmonx) (Z-EBV) is a one-way silicon valve set in a self-expanding nitinol mesh; calibration is performed using a system of sizing wings of varying sizes to determine the bronchus diameter. We generally used IBV valves, except in 1 patient in whom a Z-EBV valve was used due to lack of availability of the other at the time of the procedure.
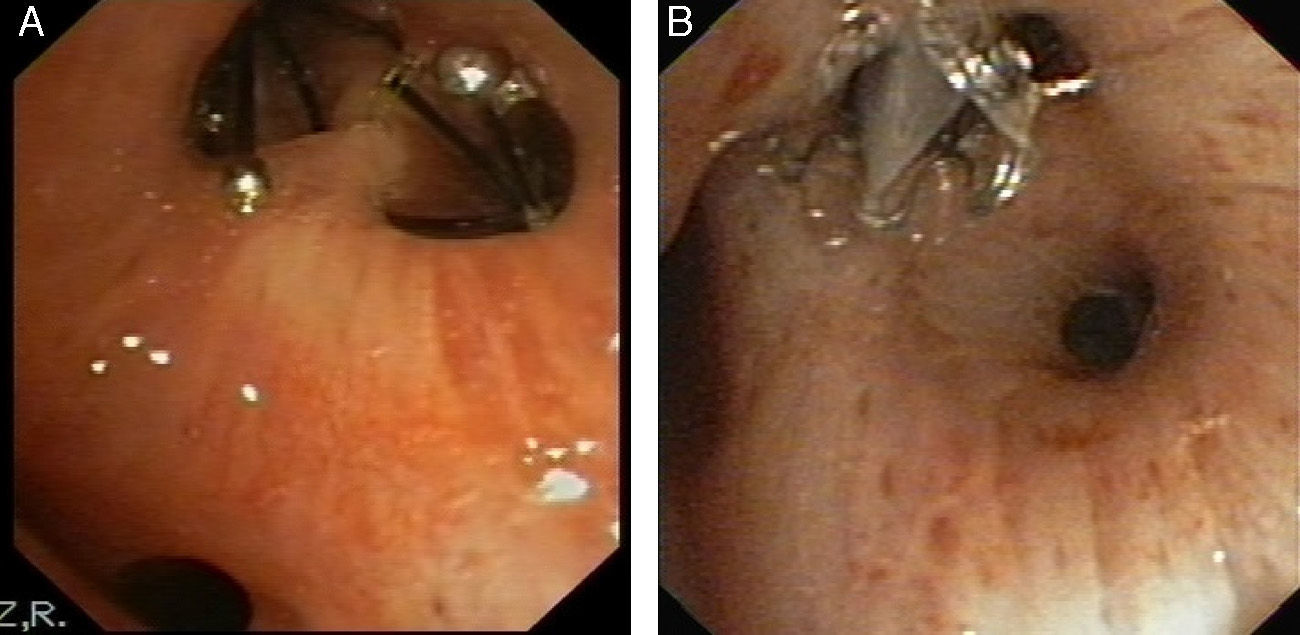
The valve is inserted via a catheter through the working channel of the bronchoscope. It self-expands on release and anchors in the bronchial mucosa (Fig. 2A and B).
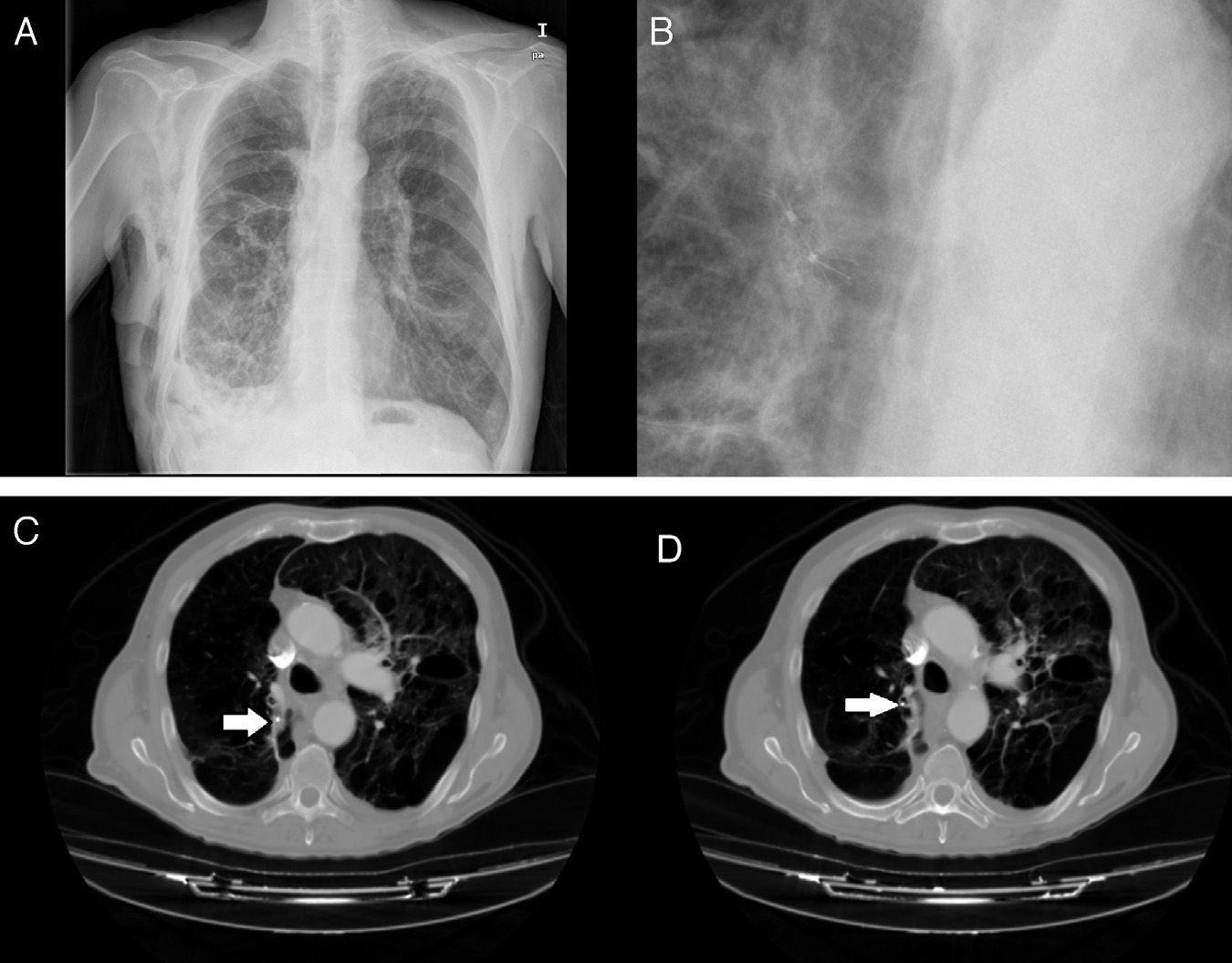
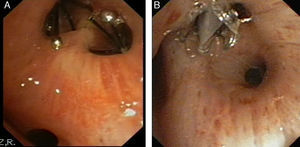
The level of anesthesia and vital signs are closely monitored throughout the procedure, and an X-ray is obtained after completion of valve placement (Fig. 3C and D) or computed tomography, if necessary, to rule out complications, confirm lung re-expansion and correct anchorage.
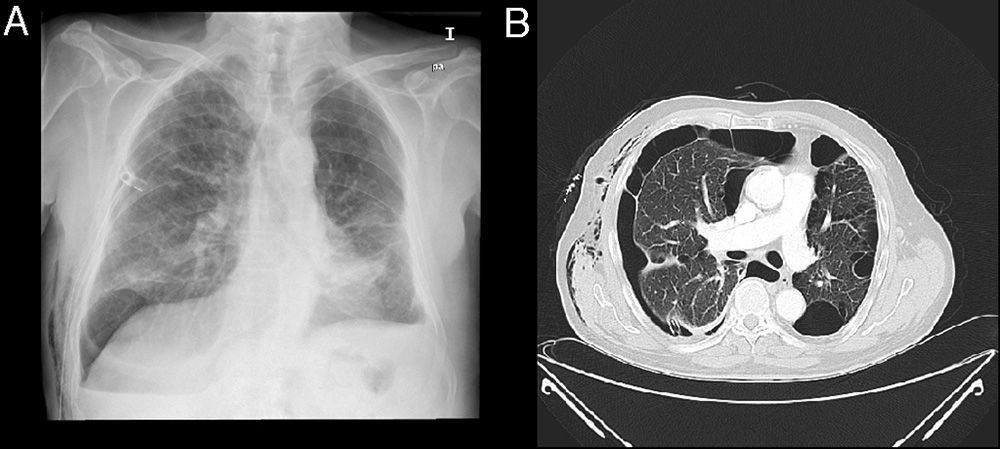
Images obtained after IBV valve placement (patient no. 8). (A) Chest X-ray with IBV valves in right upper lobe. (B) Detail of chest X-ray with IBV valves in right upper lobe. (C) Chest X-ray in patient with bullous emphysema and IBV valve in right B2 (indicated by the arrow). (D) Chest X-ray in patient with bullous emphysema and IBV valve in right B1 (indicated by the arrow).
The procedure is defined as successful if the air leak is completely stopped and the chest tube can be removed.
Valve RemovalWhen the PAL episode has resolved and the chest tube has been removed, after about 6 weeks both types of valve are removed with flexible bronchoscopy in the procedure room. Patients were followed up for up to 3 months after the procedure to monitor for possible complications derived from the implant (pneumonia, bronchial colonization, valve migration, hemoptysis, etc.), and for evaluation of recurrent pneumothorax.
ResultsTwo patients were excluded from the study after the origin of the PAL could not be located with the occlusion procedure.
Nine procedures were performed in 8 patients with PAL. The series consisted of 7 men and 1 woman, with a mean age of 68.5 years. All patients has significant comorbidities, including severe pulmonary emphysema in 7 patients, respiratory failure in 5, thrombopenia <20000 platelets per microliter in 1 and ischemic heart disease in 1 patient. In 6 patients, PAL occurred after development of a spontaneous pneumothorax secondary to underlying lung disease (Fig. 4A and B), after treatment-related pneumothorax in 1 and after anatomical resection of lung cancer in 1.
Air leak was continuous in 5 cases and intermittent in the other 3. On average, 2 valves were placed per patient (IBV in 7 and Z-EBV in 1), with a mean pleural drainage time before insertion of 15.5 days. One patient with secondary spontaneous pneumothorax (case no. 8) did not initially respond to valve placement in the right lower lobe. The patient underwent surgery, but this was also ineffective, so a new EBV was placed in the right upper lobe and the clinical picture was finally resolved.
No complications were recorded during the procedure, mean time of pleural drainage after valve placement was 13 days and leakage was stopped in 6 patients, giving a success rate of 75% in our patients. There were no adverse events associated with the implant during the 3 months of follow-up.
The valves were left in place for a median time of 52.5 days, although in 1 case it was not removed until 2 years later, as the patient failed to attend the follow-up visit. Patient characteristics are described in Table 1.
Description of Patients and Procedures.
| Characteristics of patients | |||||||||
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8a | |
| Age | 55 | 85 | 66 | 28 | 69 | 74 | 72 | 68 | |
| Sex | Male | Male | Male | Female | Male | Male | Male | Male | |
| Diagnosis | Silicosis Post TB | COPD Silicosis | COPD Bulla | Post TB | COPD | Post-resection | COPD | COPD | |
| Days of previous drainage | 15 | 16 | 14 | 90 | 22 | 22 | 8 | 12 | 6 |
| Site of air leak | RLL | Lingula | RUL | LUL | RUL | LLL | LLL | RLL | RUL |
| Type of air leakI: intermittent/C: continuous | I | C | C | I | C | C | I | C | C |
| No. of valves | 4 | 1 | 2 | 3 | 2 | 1 | 2 | 1 | 2 |
| Size of valve (mm) | 6 α 7 | 6 | 6 | 6 | 4 | 7 | 7 | 6 | 7 |
| Type of valve | IBV | IBV | IBV | IBV | Z-EBV | IBV | IBV | IBV | IBV |
| Days of drainage after EBV | 14 | 4 | 4 | 20 | 12 | 15 | 4 | 14 | 15 |
| Treatment success | No | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Surgery after EBV | Yes | No | Yes | No | No | No | No | Yes | No |
| Valve extraction (days) | 19 | 56 | 60 | 720 | 49 | 39 | 40 | 8 | 65 |
| Relapse | No | No | No | Yes | No | No | No | Yes | No |
EBV: endobronchial valve. IBV: Olympus®/Z-EBV: Zephyr®.
In this paper we report the largest series of patients with PAL treated with implantation of endobronchial valves published to date in Spain. This treatment was effective in secondary pneumothorax and pneumothorax after lung resection. Complete cessation of leakage in our patients was achieved in 75% of the cases, with no need for other procedures after withdrawal of the chest tube.
Relatively few studies in the literature have addressed this issue. In general, retrospective case series with a small heterogeneous population in terms of patient characteristics and air leak etiology have been described. The first case of a PAL successfully treated with endobronchial valves in the world literature was published in 2006 by Feller-Kopman et al.12 in Spain, the only publication on this subject was that of Rosell et al.13 which appeared in 2010. The largest series are those published by Travaline et al.14 with 40 patients, reporting a success rate of 48% and by Firlinger et al.15 in 2013, reporting on 16 patients with a success rate of 76.9%. Other publications report case series of between 1 and 7 patients.8,9,12,13,16–23
These articles were reviewed, and without taking into account the heterogeneity of the sample, the use of valves has been reported in 90 patients. Complete resolution of air leak in 63% of these patients (57 patients) and partial resolution in 28% (26 cases) was achieved. Mean number of valves used in all the reports was 3.
In general terms, all these reports present EBV as a valid alterative for the treatment of PAL, coinciding with the findings of our study. However, ideal candidates for this treatment cannot be clearly identified from these results. In our series, 3 patients had to undergo surgery, despite their severe comorbidities, as the air leak was not completely resolved after EBV placement. The first of these underwent an intervention 14 days after valve placement. In this patient, the occlusion test may have been unsuccessful as the air leak, being intermittent, was difficult to identify. In the second patient, the air leak was so severe that despite EBV treatment, the decision was taken to operate on day 5. In the third case (case no. 8), surgery after valve placement was unsuccessful, so a second non-invasive procedure was performed for placement of new EBV that were effective in stopping the air leak.
When PAL is intermittent, it can be difficult to locate the air leak using the occlusion test, particularly if it is not completely stopped and only some reduction is observed. Firlinger et al.15 excluded patients with intermittent leak from their study, and this would explain the greater success rate in their series compared to the figures published in the literature. Moreover, they used a digital measurement system that may have helped in the selection of patients for this type of treatment. In our series, a similar success rate was obtained, despite the inclusion of 3 cases with intermittent air leak.
Since the series was small, it is difficult to determine reasons for response or non-response to EBV, suggesting that prospective studies and analyses of predictors for success in this type of treatment would be of interest: type and volume of PAL, age, cause of leak, previous lung function, body mass index, etc.
Extrapolating from the work of Wan et al.24 in the context of volume reduction treatment, possible complications of the procedure include pneumonia, empyema, hemoptysis, and respiratory failure. Mortality is low and associated more with the underlying disease than the procedure. Jenkins et al.25 also described valve migration as a possible complication and in the series published by Travaline et al.14 patients with incorrect valve positioning, expectoration of a valve, moderate desaturation, pneumonia, and colonization of the valve with methicillin-resistant Staphylococcus aureus were reported. Another significant complication mentioned in the literature is dyspnea and respiratory failure due to bronchial occlusion in patients with emphysema and with prior lung resection for lung cancer. In this respect, Dooms et al.26 described a case in which, after right lower lobectomy, occlusion of the bronchi of the right upper lobe produced no atelectasis of that lobe, probably due to collateral ventilation. However, collateral ventilation does not prevent leakage closure after valve insertion, since the valve itself reduces the passage of air, allowing the lung to heal. No atelectasis was found in our series, so none of the patients experienced increased dyspnea or oxygen desaturations after valve placement. Moreover, development of atelectasis over time was not expected as full lobe occlusion was not performed in any of our cases.
To sum up, in our series the procedure was safe and well-tolerated and free of complications. No complications developed during the 3 months of follow-up, although the youngest patient had a recurrence, in the form of a small apical chamber that did not require drainage and was resolved with respiratory physiotherapy. As in other studies, the limitations of a lack of control group, the small sample size and the range of causes of air leak must be mentioned.
ConclusionTreatment of persistent air leak with endobronchial valves is minimally invasive, effective, and safe. It is a good alternative for patients with high surgical risk. The results of our series are similar to those published by other groups of investigators, and the study presents the same weaknesses: it is retrospective, the sample size is small, and the causes of PAL are heterogeneous. Well-designed prospective studies are needed to confirm the indications for EBVs and factors predictive of success.
Authors’ ContributionDr. Cordovilla was responsible for conducting procedures, study concept and design, data collection, analysis and interpretation, drafting the manuscript and review of its intellectual content and review of the final version submitted for evaluation.
Dr. Torracchi participated in conducting the procedures, study concept and design, data collection, analysis and interpretation, drafting of the manuscript and review of its intellectual content, and review of the final version submitted for evaluation.
Dr. Varela participated in conducting the procedures and in the review of its intellectual content and review of the final version submitted for evaluation.
Dr. Jiménez participated in conducting the procedures and in the review of its intellectual content and review of the final version submitted for evaluation.
Dr. Aranda participated in conducting the procedures and in the review of its intellectual content and review of the final version submitted for evaluation.
Dr. Novoa participated in conducting the procedures and in the review of its intellectual content and review of the final version submitted for evaluation.
Dr. Barrueco contributed to the review of the intellectual content of the manuscript and review of the final version submitted for evaluation.
Conflicts of InterestsThe authors do not have any conflict of interests.
Our thanks to Manuel Lanchas Hernando, Mª José Rodríguez Celador and Emilia Pedraz Rivas, for their contribution and daily assistance during the endoscopic procedures.
Please cite this article as: Cordovilla R, Torracchi AM, Novoa N, Jiménez M, Aranda JL, Varela G, et al. Válvulas endobronquiales para el tratamiento de la fuga aérea persistente, una alternativa al tratamiento quirúrgico. Arch Bronconeumol. 2015;51:10–15.