Hemoptysis is symptomatic of potentially serious and life-threatening chest disease and requires urgent evaluation and treatment. The aim of this study was to evaluate the hemostatic efficacy of endobronchial application of Ankaferd Blood Stopper® (ABS) solution in patients with hemoptysis.
This retrospective study included 20patients with hemoptysis in whom endobronchial ABS was applied in 25bronchoscopic procedures.
Endobronchial application of ABS was successful in 23 of the 25 bronchoscopic procedures. ABS application was repeated due to recurrent bleeding in 4patients.
This is the first case series demonstrating the endobronchial application of ABS, a novel hemostatic agent, effective in the management of bleeding, especially in local endobronchial malignant lesions. Bronchoscopic ABS application may be an alternative supportive therapeutic method in cases of uncontrolled hemoptysis.
La hemoptisis es una manifestación sintomática de una enfermedad torácica que puede ser grave y poner en peligro la vida, y requiere una evaluación y tratamiento urgentes. El objetivo del presente estudio fue evaluar la eficacia hemostásica de la aplicación endobronquial de la solución Ankaferd Blood Stopper® (ABS) en pacientes con hemoptisis.
Este estudio retrospectivo se realizó en 20 pacientes con hemoptisis en los que se aplicó ABS endobronquial mediante 25broncoscopias.
La aplicación endobronquial de ABS tuvo éxito en 23 de las 25broncoscopias. Se repitió la aplicación de ABS a causa de una recidiva hemorrágica en 4pacientes.
Esta es la primera serie de casos que describe el uso de la aplicación endobronquial de ABS, un nuevo agente hemostásico que constituye un método eficaz para el tratamiento de la hemorragia, sobre todo en lesiones malignas endobronquiales locales. Pensamos que la aplicación broncoscópica de ABS puede ser un método terapéutico alternativo y de apoyo en casos de hemoptisis no controlada.
Ankaferd hemostat (Ankaferd Blood Stopper® [ABS]) is an extract of popular medicinal plants that has been used as a hemostatic agent in traditional Turkish medicine. It consists of a standard mixture of plants, all of which have antithrombin, antiplatelet, antioxidant, antiatherosclerotic and antitumor activity. The basic mechanism of action of ABS is the formation of an encapsulated protein network that provides a focus for vital erythroid aggregation.1 The paradoxical antithrombotic action of an antihemorrhagic product can be explained by its effects on gamma fibrinogen seen in functional proteomic analysis.2 ABS was recently registered for the treatment of clinical bleeds when conventional control methods are ineffective. The aim of this study was to evaluate retrospectively the efficacy of the endobronchial application of ABS hemostat in the treatment of hemoptysis.
Patients and methodsABS was used in 20 patients (18 men and 2 women) who underwent 25 local bronchoscopies with flexible fiberoptic bronchoscope (Olympus® BF XT20, BF-1T160 and Pentax® 18P). Informed consent was obtained from all patients before performing the bronchoscopy. The main indication for the use of ABS was the control of bleeding originating in the bronchial tree that persisted despite lavage of the affected segment with saline cooled in ice and/or adrenaline instillation in the bleed site. In 7 cases, bleeding began after an endobronchial or transbronchial biopsy, and in the other 13 there was diffuse or local endobronchial bleeding. If the bleeding lesion was visible, ABS was applied directly onto the bleed site via the bronchoscopic probe. If there was diffuse bleeding in a lung segment, ABS was applied from the main carina with a bronchoalveolar lavage catheter. The 2ml dose was repeated if response was insufficient. Depending on the amount of daily bleeding (hemoptysis severity), patients were assigned to 4 groups as follows,3 mild: only hematic streaks in sputum; moderate: 30-100cm3; severe: 100-600cm3; massive: > 600cm3. Successful control of bleeding after ABS application was defined as cessation of bleeding during the bronchoscopic intervention and no hemorrhagic relapse over a 24-hour period.
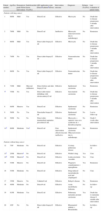
ResultsThe mean age of patients was 58 years (range, 25-81 years). Hemoptysis was the presenting symptom in 13 patients and in 7 it was bleeding after bronchoscopic biopsy. Bleeding could be controlled with ABS a few seconds after instillation in the hemorrhagic focus in 23 interventions, but it was ineffective in 2 cases. ABS was reapplied due to relapsed bleeding in 4 patients in whom hemoptysis recurred within 24hours after the first intervention. Clinical characteristics of patients are listed in Table 1.
Clinical characteristics of patients who received endobronchial ABS in the treatment of hemoptysis.
| Patient number | Age/Sex | Hemoptysis grade before intervention | Endobronchial lesion (Yes/No) | ABS application route (Directa/catheterb)/Dose: | Intervention outcome | Diagnosis | Relapse (Yes/No) | Last evaluation of patient status |
| Patients with lung cancer | ||||||||
| 1 | 69/M | Mild | Yes | Direct/2 ml | Effective | Microcytic | No | Death due to disease progression 4 months after intervention |
| 2 | 79/M | Mild | No | Direct/2 ml | Ineffective | Microcytic (mucosal erosion due to radiotherapy) | No | Remission |
| 3 | 68/M | Mild | Yes | Direct (after biopsy)/2 ml | Effective | Microcytic | No | Death due to disease progression 3 months after intervention |
| 4 | 74/M | No | Yes | Direct (after biopsy)/2 ml | Effective | Neuroendocrine tumor | No | Death due to disease progression 3 months after intervention |
| 5 | 68/M | No | Yes | Direct (after biopsy)/2 ml | Effective | Neuroendocrine tumor | No | Death due to disease progression 3 months after intervention |
| 6 | 73/M | Mild | Mucosal infiltration | Direct (before and after biopsy)/2 ml | Effective | Epidermoid carcinoma | Yes | Remission |
| 7c | 75/M | No | Yes | Direct (after tumor debulking with electrocautery)/2 ml | Effective | Sarcomatoid carcinoma | No | Death due to disease progression 5 months after intervention |
| 8 | 64/M | Massive | Yes | Direct/2 ml | Effective | Epidermoid carcinoma | No | Remission |
| 9 | 58/M | No | Yes | Direct(after biopsy)/2 ml | Effective | Epidermoid carcinoma | No | Lost to follow-up |
| 10 | 59/M | No | No | Direct (after transtracheal aspiration procedure)/2 mL | Effective | Microcytic + superior vena cava syndrome de + thrombocytopenia | No | Death 2 weeks after intervention |
| 11d | 60/M | Moderate | Yese | Direct/2 ml | First intervention effective/second ineffective | Epidermoid carcinoma /Mucosal erosion due to radiotherapy | No | Remission |
| Patients without lung cancer | ||||||||
| 12 | 75/F | Moderate | No | Direct/2 ml | Effective | Cavitary pulmonary disease | In follow-up | |
| 13f | 25/M | Massiveg | No | Direct/2 ml | Effective | Welder's lung | Yes | Cure |
| 14h | 55/M | Massiveg | No | Direct/2 ml | Effective | Leukocytoclastic vasculitis | Yes | Cure |
| 15 | 53/M | Massive | No | Direct/2 ml | Effective | Wegener's granulomatosis + aspergilloma | Yes | Remission |
| 16 | 53/M | Moderate | No | Direct/2 ml | Effective | Drug-induced (acetylsalicylic acid and clopidogrel) | No | Cure |
| 17 | 35/M | Massive | No | Catheter/4 ml | Effective | Behçet's disease | No | Remission |
| 18 | 40/M | Moderate | No | Direct/4 ml | Effective | Acute myeloblastic leukemia | No | In follow-up |
| 19 | 81/M | Moderate | Yes | Direct/2 ml | Effective | Pulmonary metastatic prostate carcinoma | Yes | Death due to metastatic disease 6 months after intervention |
| 20 | 45/F | No | No | Direct (after biopsy)/2 ml | Effective | Rheumatoid arthritis + Bronchiolitis | No | In follow-up |
ABS: Ankaferd Blood Stopper®; F: female; M: male.
Eight patients had hemorrhagic endobronchial lesions due to lung cancer, with tumor infiltration of the mucosa in one. Bleeding began after transbronchial biopsy in one patient and after a transtracheal aspiration procedure in another.
In 9 of the patients, hemoptysis was due to non-malignant causes (Table 1). Six patients had massive bleeding (5 in the non-malignant group and one in the malignant group). Vasculitis was the most common cause of massive bleeds.
DiscussionThe aim of this study is to describe an alternative therapeutic option in emergency settings using the endobronchial application of ABS. Endobronchial application of a dose of 2-4ml ABS was found to be effective in most situations. Due to the fast action of ABS, hemorrhage can be controlled in a few seconds after application. This drug was particularly effective in local hemorrhagic lesions. Only one case of endobronchial application of ABS for the control of a malignant endobronchial lesion has been described.4 However, the topical administration of ABS has been successfully tested in many other bleeding situations, such as gastrointestinal hemorrhage, epistaxis, hemorrhages in hemophiliacs, dental bleeds and post-tonsillectomy bleeding.5 Lung cancer was the underlying cause of the majority of cases in this series (11 patients) and in 7 cases, bleeding began after biopsy. The endobronchial application of ABS stopped bleeding in all cases, thus preventing unnecessary diagnostic and curative delays in patient management. In this study, there were 5 cases of massive hemoptysis in patient groups without cancer. In all these patients, ABS was effective in the treatment of pulmonary bleeding. Moreover, immunosuppressive treatment in vasculitis and supportive treatment in hemoptysis due to welding fumes improved clinical and radiological results.
To conclude, this is the first case series describing the use of endobronchial application of ABS, a new hemostatic treatment representing an effective method for the treatment of bleeding, particularly in local endobronchial malignant lesions. Consequently, bronchoscopic application of ABS may be an alternative supportive treatment in cases of uncontrolled hemoptysis. More controlled, prospective clinical studies will be necessary to fully identify the safety, dosage regimen and efficacy of the topical use of ABS in patients with hemoptysis.
Please cite this article as: Uzun O, Erkan L, Haznedaroglu IC. Tratamiento efectivo de la hemoptisis mediante aplicación endobronquial del hemostásico Ankaferd. Arch Bronconeumol. 2014;50:407–409.