High-flow nasal cannula (HFNC) is a non-invasive respiratory support modality that provides heated and fully humidified gas mixtures to patients thorough a nasal cannula interface.1 In pediatrics, HFNC is primarily used in a hospital setting for patients with bronchiolitis, and data on its domiciliary use has been limited.2 CHARGE syndrome is a congenital syndrome characterized by coloboma, heart malformation, choanal atresia, retarded growth and development, genital hypoplasia, and ear anomalies/deafness with recurrent respiratory infections.3,4 We describe a patient with CHARGE syndrome in whom the frequency of hospitalization due to respiratory infections was successfully reduced by domiciliary HFNC.
The patient was a male baby born at 37 weeks via cesarean section, which was performed because of a dichorionic diamniotic twin pregnancy, and his 1- and 5-min Apgar scores were 4 and 8, respectively. His birth weight was 2738g (−0.7 standard deviation [SD]), and height was 44.5cm (−2.1 SD). He had cryptorchidism, micropenis, and an auricular malformation. He also had dysphagia and needed a nasogastric tube for feeding. Transthoracic echocardiography revealed a persistent patent ductus arteriosus and atrial septal defect. At the age of three months, auditory brainstem response test did not reveal wave V, and magnetic resonance imaging of the internal auditory canal showed hypoplasia of the semicircular canals and deficient cochlear nerves bilaterally. Coloboma was not detected by ophthalmoscopy. The presence of pathogenic chromodomain helicase DNA-binding protein-7 variants could not be examined because his parents refused the analysis. The patient was diagnosed with atypical CHARGE syndrome according to the Verloes's diagnostic criteria.4
At the age of 6 months, the patient was admitted to our hospital for bronchiolitis and introduced nasopharyngeal suctioning and biphasic cuirass ventilation (Hayek RTX, Medivent, London, UK); however, he continued to be hospitalized once or twice a month for lower respiratory infections.
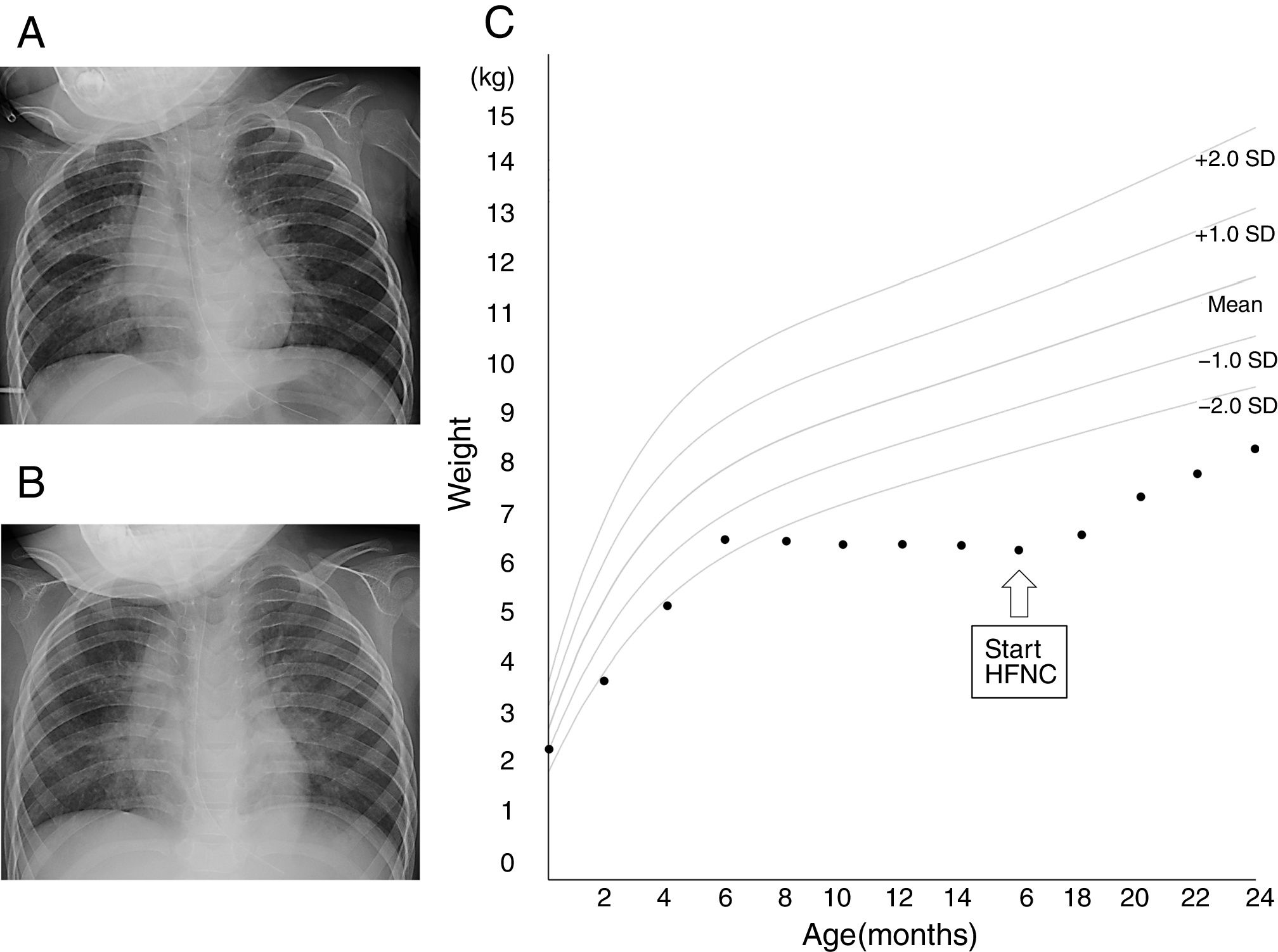
At the age of 16 months, the patient was admitted to the emergency department because of fever and productive cough. His percutaneous oxygen saturation was lower than 90%, while breathing room air. Analysis of the venous blood gas revealed the following: pH, 7.35; bicarbonate, 24.3mmol/L; and partial pressure of carbon dioxide, 44.5mmHg. Chest X-ray (CXR) showed consolidation in the right middle and lower lung fields (Fig. 1A), which was considered as atelectasis; the consolidation in these areas was present in a prior CXR obtained at the age of 13 months. He was diagnosed with bronchiolitis and hospitalized to start treatment with ampicillin (180mg/kg/day, every 8h), low-flow oxygen delivery via a nasal cannula, and inhalation therapy with a short-acting β2 agonist. However, tachypnea and wheezing persisted. On hospital day 10, the HFNC therapy was introduced using a myAIRVO™2 device with an Optiflow™ junior nasal interface (Fisher & Paykel, Auckland, New Zealand), which was set to temperature of 34̊C, flow rate of 18L/min (2.9L/kg/min), and 2L of oxygen, the equivalent to 0.3 of fraction of inspired oxygen (FiO2). After HFNC initiation, tachypnea and wheezing subsided, and he was discharged on hospital day 16 with continued at-home HFNC use for respiratory support during sleep. He used HFNC for an average of 12h per day. Written informed consent was obtained from his mother to use HFNC in a domiciliary setting and for publication of the patient's information. The settings of HFNC were changed to a flow rate of 8L/min (1.3L/kg/min) and 2L of oxygen, which corresponds to 0.39 of FiO2. After discharge from the hospital, the patient was treated with clarithromycin (5mg/kg/day) and pranlukast (7mg/kg/day).
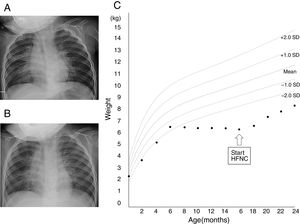
Chest X-ray images before the initiation of high-flow nasal cannula (A) and 1 month (B) after the initiation of high-flow nasal cannula. (C) Growth chart of the patient. Source: Ref. 5.
One month after the domiciliary HFNC initiation, a CXR showed an improvement of the consolidation (Fig. 1B), and the patient did not require hospitalization for respiratory infections for ten months after the domiciliary HFNC initiation. Importantly, the patient did not experience any adverse reactions associated with HFNC, such as pneumothorax, aerophagia, akin irritation, or epistaxis.
According to the growth standard charts for Japanese children,5 his weight gain was along the −2.0 SD line for the first 6 months; however, over the next 10 months, his weight dropped from 6.6kg (−1.6 SD) to 6.3kg (−3.6 SD). After the domiciliary HFNC initiation, his weight increased to 8.3kg (−2.7 SD) at the age of 24 months (Fig. 1C).
This case demonstrates three beneficial effects of domiciliary HFNC in the absence of any adverse effects in a patient with CHARGE syndrome: improvement of atelectasis, decrease in hospitalization frequency, and significant weight gain. However the potential contribution of clarithromycin and pranlukast to the patient's clinical course cannot be ruled out.
HFNC generates a positive end-expiatory pressure, which can prevent alveolar closure, and improves mucociliary clearance.6 These beneficial effects might have led to the improvement of atelectasis in our patient.
Two reports showed that long-term domiciliary humidification via HFNC reduced the number of exacerbation days and hospital admission rates and improved lung function in adult patients with chronic obstructive pulmonary disease and bronchiectasis.7,8 These outcomes might be attributable to an improvement of small airway function and reduction in gas trapping through the improvement of mucociliary clearance.7–9 In agreement with the previous reports, these effects of HFNC were considered to have contributed to the decreased hospitalization frequency in our patient.
Impairment of weight gain reflects an imbalance between dietary intake and energy expenditure. The dietary support in our patient was provided entirely by a nasogastric tube. HFNC lowers energy expenditure by decreasing work of breathing via the reduction of inspiratory resistance and wash out of nasopharyngeal anatomical dead space.6 Therefore, the significant weight gain in this patient might have been due to the improvement in imbalance between dietary intake and energy expenditure.
This case highlights domiciliary HFNC as a promising, noninvasive respiratory support option for the management of children with chronic respiratory problem. Future studies with a greater number of pediatric participants are required to evaluate the clinical effect and safety and elucidate the potential mechanisms of domiciliary HFNC.
FundingThis research did not receive any specific grant from funding agencies in the public commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to Dr. Nishijima, Dr. Kanda, and Dr. Abe for participating in the care.