Although the major limitation to exercise performance in patients with COPD is dynamic hyperinflation (DH), little is known about its relation with cardiac response to exercise. Our objectives were to compare the exercise response of stroke volume (SV) and cardiac output (CO) between COPD patients with or without DH and control subjects, and to assess the main determinants.
MethodsFifty-seven stable COPD patients without cardiac comorbidity and 25 healthy subjects were recruited. Clinical evaluation, baseline function tests, computed tomography and echocardiography were conducted in all subjects. Patients performed consecutive incremental exercise tests with measurement of operating lung volumes and non-invasive measurement of SV, CO and oxygen uptake (VO2) by an inert gas rebreathing method. Biomarkers of systemic inflammation and oxidative stress, tissue damage/repair, cardiac involvement and airway inflammation were measured.
ResultsCOPD patients showed a lower SV/VO2 slope than control subjects, while CO response was compensated by a higher heart rate increase. COPD patients with DH experienced a reduction of SV/VO2 and CO/VO2 compared to those without DH. In COPD patients, the end-expiratory lung volume (EELV) increase was related to SV/VO2 and CO/VO2 slopes, and it was the only independent predictor of cardiac response to exercise. However, in the regression models without EELV, plasma IL-1β and high-sensitivity cardiac troponin T were also retained as independent predictors of SV/VO2 slope.
ConclusionDynamic hyperinflation decreases the cardiac response to exercise of COPD patients. This effect is related to systemic inflammation and myocardial stress but not with left ventricle diastolic dysfunction.
Aunque la principal limitación para el rendimiento durante el ejercicio en pacientes con EPOC es la hiperinsuflación dinámica (HD), se sabe poco sobre su relación con la respuesta cardíaca al ejercicio. Nuestros objetivos fueron comparar la respuesta al ejercicio del volumen sistólico (VS) y el gasto cardíaco (GC) entre los pacientes con EPOC con o sin HD y sujetos control, y evaluar los principales determinantes.
MétodosSe reclutaron 57 pacientes con EPOC estable sin comorbilidad cardíaca y 25 sujetos sanos. En todos los sujetos se realizó una evaluación clínica, pruebas de función basal, una tomografía computarizada y una ecocardiografía. Los pacientes realizaron pruebas de esfuerzo incrementales consecutivas con medición de los volúmenes pulmonares operativos y medición no invasiva del VS, el GC y el consumo de oxígeno (VO2) mediante un método de reinhalación de gas inerte. Se midieron los biomarcadores de inflamación sistémica y estrés oxidativo, daño/reparación tisular, afectación cardíaca e inflamación de las vías respiratorias.
ResultadosLos pacientes con EPOC presentaron una curva más baja de VS/VO2 que los controles, mientras que la respuesta del GC se compensó con un mayor aumento del ritmo cardíaco. Los pacientes con EPOC e HD experimentaron una reducción de VS/VO2 y de GC/VO2 en comparación con aquellos sin HD. En los pacientes con EPOC, el aumento del volumen pulmonar teleespiratorio (EELV, por sus siglas en inglés) se relacionó con las curvas de VS/VO2 y GC/VO2, y fue el único factor predictivo independiente de la respuesta cardíaca al ejercicio. Sin embargo, en los modelos de regresión sin EELV, la IL-1β plasmática y la troponina T cardíaca ultrasensible también se mantuvieron como factores predictivos independientes de la curva de VS/VO2.
ConclusiónLa hiperinsuflación dinámica disminuye la respuesta cardíaca al ejercicio en los pacientes con EPOC. Este efecto se relaciona con la inflamación sistémica y el estrés miocárdico, pero no con la disfunción diastólica del ventrículo izquierdo.
Chronic obstructive pulmonary disease (COPD) is a progressive life-limiting condition and a major cause of mortality worldwide.1 COPD affects about 328 million people worldwide, and it is the third leading cause of death, accounting for 3.2 million deaths each year.2 Many of these deaths are related to cardiovascular morbidity, especially in patients with mild-to-moderate COPD.3 Pathophysiological links between COPD and cardiovascular disease include lung hyperinflation, hypoxemia, pulmonary hypertension, systemic inflammation and oxidative stress, exacerbations, shared risk factors and shared genetics, as well as COPD phenotype.4
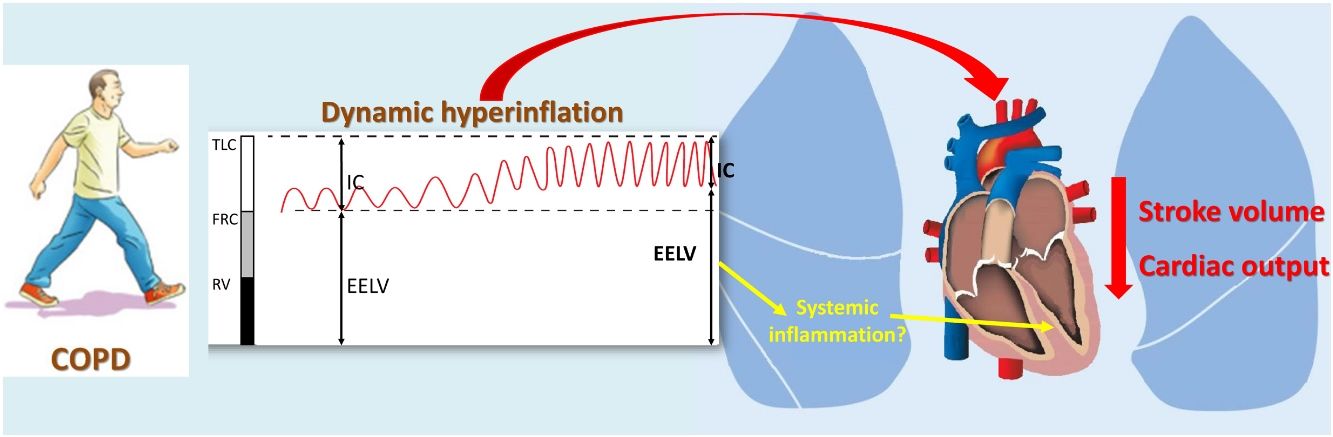
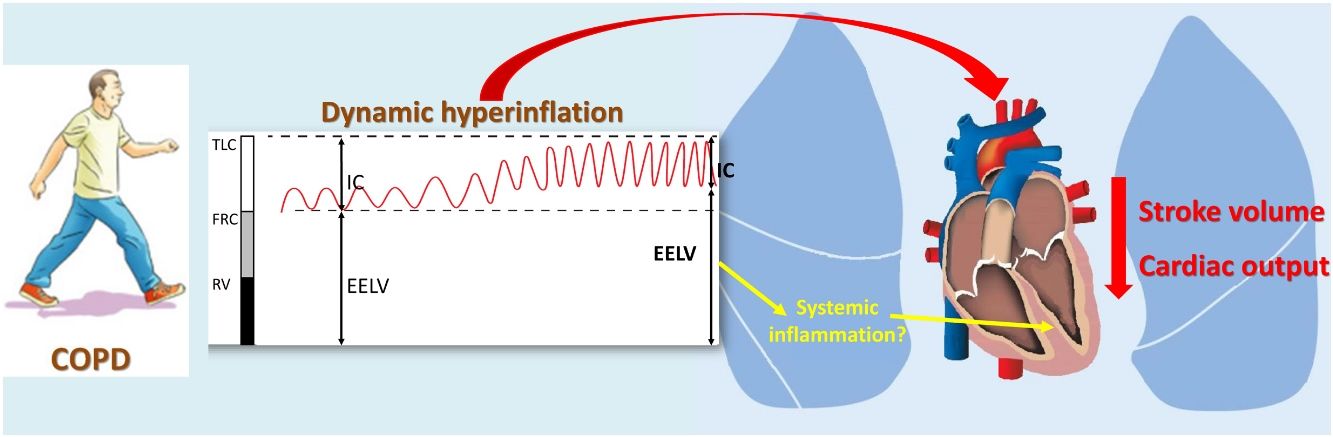
Dynamic hyperinflation (DH) is a frequent pathophysiological disorder experienced by many COPD patients that is a main determinant of symptom perception,5 exercise tolerance6 and daily physical activity.7 Although the association between lung hyperinflation and reduced cardiac function has received much attention in recent years,8–10 the effect of DH on cardiac response to exercise remains less known. Most of the available information comes from classical studies on the effect of exercise and voluntary hyperventilation on cardiac function as well as the extrapolation of studies conducted in patients with mechanical ventilation.11,12 However, the demonstration of a potential effect of DH on the cardiac response to exercise could establish a link between COPD and cardiovascular morbidity, mainly in the early stages of the disease, when there is still no developed cardiac structural damage.
To our knowledge, only one study to date has simultaneously evaluated the DH and cardiac response to exercise of COPD patients, although using surrogated outcomes. In 45 COPD patients, Tzani et al.13 analyzed the relationship between end-expiratory lung volume (EELV) increase and cardiac response, assessed by oxygen pulse and the product of systolic blood pressure and heart rate (DP reserve), reporting that the increase in EELV maintained a negative relationship with both parameters. Although interpretation must be done cautiously because there are indirect measures, the lower response of oxygen pulse in patients with DH could be attributed to a lower preload due to a diastolic filling defect of the left ventricle (LV), whereas the lower response of the DP reserve might suggest impaired LV contractility.13 This is particularly interesting since DH promotes low-grade systemic inflammation in stable COPD patients.14 Moreover, it is known that subcellular low-grade inflammation in the heart induces subcellular component abnormalities, such as oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress and impaired calcium handling, leading to impaired myocardial contractility.15
Therefore, our objective was to compare the response of stroke volume (SV) and cardiac output (CO) to exercise between COPD patients with or without DH and control subjects. Moreover, we have tried to evaluate the main determinants of this response, primarily considering lung parenchymal damage, left ventricular systolic and diastolic function, and biomarkers of systemic inflammation and oxidative stress, heart tissue damage and airway inflammation.
MethodsStudy subjectsFifty-seven stable COPD patients with no cardiac comorbidity were consecutively recruited, and a control group of 25 healthy subjects was randomly selected from our laboratory's reference group. The study was approved by the local ethics committee, and informed consent was given by all subjects. Additional details about the selection criteria are provided in the online supplementary data.
Clinical evaluationBody composition, smoking habits, baseline dyspnea level, Charlson comorbidity index, health-related quality of life (St. George's Respiratory Questionnaire) and daily physical activity (International Physical Activity Questionnaire) were collected in all subjects. Additionally, we recorded for COPD patients the following: GOLD risk group, BODE and ADO multidimensional indices, current treatment and COPD Assessment Test (CAT). Arterial blood gas values breathing room air, spirometry, lung volumes and diffusing capacity were measured according to current recommendations,16–18 using as reference values the Global Lung Function Initiative19,20 and European Coal and Steel Community21 equations. A duplicate 6-min walk test was carried out in accordance with the guidelines of the American Thoracic Society.22
Biomarkers and imaging techniquesA panel of plasma biomarkers related to inflammation, oxidative stress, tissue damage/repair and cardiac involvement was determined (Table S1). In addition, biomarkers of airway inflammation were measured in exhaled breath condensate (EBC). Further details on the method used for making these measurements are provided in the online supplementary data.
From computed tomography examinations at maximal inspiration and expiration, attenuation of lung parenchyma was assessed by a semiautomatic analysis program. Transthoracic echocardiography with tissue Doppler imaging was performed in accordance with current recommendations. A more detailed description of both procedures is provided in the online supplementary data.
Exercise testing and cardiac response to exerciseA symptom-limited incremental exercise test was conducted on a cycle-ergometer following ATS/ACCP standards.23 Workload was increased by 15W/min, and expired gases, ventilation, and 12-lead electrocardiogram were continuously measured (Oxycon Alpha, Viasys, Hoechberg, Germany).24 The predicted values by Jones were used.25 Anaerobic threshold was estimated using the nadir of the ventilatory equivalent and the V-slope method.23
At least two reproducible inspiratory capacity maneuvers were obtained at rest and every 2min during exercise, and the mean end-expiratory lung volumes (EELV) of the three preceding breaths were determined.26 To minimize the variability of isolated EELV measurements, the patient was considered to have developed DH when the slope of linear regression of the EELV as a function of time was greater than zero.7
On a following day, other incremental cycle exercise tests were performed, measuring the SV and CO at the end of the resting period, at 15W, and at 45W, using an inert gas rebreathing method (Innocor, Innovision, Odense, Denmark).27 Expired gas analysis was performed continuously throughout the test with the Innocor system, which uses an oxygen-enriched mixture of an inert soluble gas (0.5% nitrous oxide) and an inert insoluble gas (0.1% sulfur hexafluoride) from a 4-L prefilled anesthesia bag, whereas a photoacoustic analyzer measured gas concentrations over a 5-breath interval. Cardiac response to exercise was assessed through the slopes of ΔSV, ΔCO and cardiac index (CI) with respect to oxygen uptake (ΔVO2), calculated by linear regression of the three measurements.
Statistical analysisValues are expressed as mean±standard deviation, median and interquartile range or percentage, according their type and distribution. Comparisons between groups were performed by the Student's t, Mann–Whitney or chi-square tests. The relationships between variables were determined using Pearson's correlation. Significant contributors to cardiac response to exercise were then introduced in a stepwise multiple linear regression analysis to identify independent determinants of the SV/VO2 slope. Statistical significance was assumed for P<0.05.
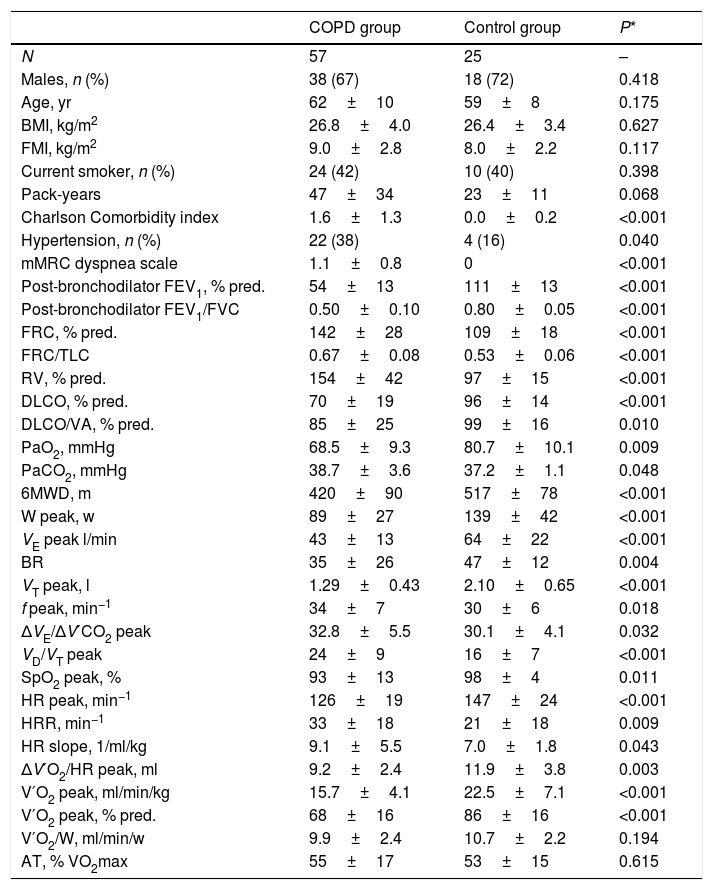
ResultsTable 1 presents the main characteristics of COPD patients and control subjects. Both groups were homogeneous in anthropometric characteristics and smoking habit, showing obvious differences in lung function and exercise tolerance. The comorbidity burden and dyspnea level of COPD patients was relatively mild, reflecting the predominance of patients with moderate airflow limitation (67%). In fact, although COPD patients had a worse SGRQ score than control subjects (40.2±16.8 vs. 15.2±8.6, P<0.001), the percentage of subjects with a moderate-high level of daily physical activity was identical in the two groups (82%). Tables S2–S4 present the comparisons between COPD patients and control subjects for the attenuation densities of the lung parenchyma, levels of systemic and airway biomarkers, and echocardiographic parameters.
General characteristics of the study subjects.
| COPD group | Control group | P* | |
|---|---|---|---|
| N | 57 | 25 | – |
| Males, n (%) | 38 (67) | 18 (72) | 0.418 |
| Age, yr | 62±10 | 59±8 | 0.175 |
| BMI, kg/m2 | 26.8±4.0 | 26.4±3.4 | 0.627 |
| FMI, kg/m2 | 9.0±2.8 | 8.0±2.2 | 0.117 |
| Current smoker, n (%) | 24 (42) | 10 (40) | 0.398 |
| Pack-years | 47±34 | 23±11 | 0.068 |
| Charlson Comorbidity index | 1.6±1.3 | 0.0±0.2 | <0.001 |
| Hypertension, n (%) | 22 (38) | 4 (16) | 0.040 |
| mMRC dyspnea scale | 1.1±0.8 | 0 | <0.001 |
| Post-bronchodilator FEV1, % pred. | 54±13 | 111±13 | <0.001 |
| Post-bronchodilator FEV1/FVC | 0.50±0.10 | 0.80±0.05 | <0.001 |
| FRC, % pred. | 142±28 | 109±18 | <0.001 |
| FRC/TLC | 0.67±0.08 | 0.53±0.06 | <0.001 |
| RV, % pred. | 154±42 | 97±15 | <0.001 |
| DLCO, % pred. | 70±19 | 96±14 | <0.001 |
| DLCO/VA, % pred. | 85±25 | 99±16 | 0.010 |
| PaO2, mmHg | 68.5±9.3 | 80.7±10.1 | 0.009 |
| PaCO2, mmHg | 38.7±3.6 | 37.2±1.1 | 0.048 |
| 6MWD, m | 420±90 | 517±78 | <0.001 |
| W peak, w | 89±27 | 139±42 | <0.001 |
| VE peak l/min | 43±13 | 64±22 | <0.001 |
| BR | 35±26 | 47±12 | 0.004 |
| VT peak, l | 1.29±0.43 | 2.10±0.65 | <0.001 |
| f peak, min−1 | 34±7 | 30±6 | 0.018 |
| ΔVE/ΔV′CO2 peak | 32.8±5.5 | 30.1±4.1 | 0.032 |
| VD/VT peak | 24±9 | 16±7 | <0.001 |
| SpO2 peak, % | 93±13 | 98±4 | 0.011 |
| HR peak, min−1 | 126±19 | 147±24 | <0.001 |
| HRR, min−1 | 33±18 | 21±18 | 0.009 |
| HR slope, 1/ml/kg | 9.1±5.5 | 7.0±1.8 | 0.043 |
| ΔV′O2/HR peak, ml | 9.2±2.4 | 11.9±3.8 | 0.003 |
| V′O2 peak, ml/min/kg | 15.7±4.1 | 22.5±7.1 | <0.001 |
| V′O2 peak, % pred. | 68±16 | 86±16 | <0.001 |
| V′O2/W, ml/min/w | 9.9±2.4 | 10.7±2.2 | 0.194 |
| AT, % VO2max | 55±17 | 53±15 | 0.615 |
Definition of abbreviations: BMI, body mass index; FMI, fat mass index; mMRC, modified Medical Research Council; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FRC, functional residual capacity; TLC, total lung capacity; RV, residual volume; DLCO, carbon monoxide diffusing capacity; VA, alveolar volume; PaO2, arterial oxygen pressure; PaCO2, carbon dioxide arterial pressure; 6MWD, 6-minute walk distance; W, work intensity; VE, minute ventilation; BR, breathing reserve; VT, tidal volume; f, respiratory frequency; ΔVE/ΔV′CO2, ventilatory equivalent for carbon dioxide; VD/VT, ratio of physiologic dead space to tidal volume; SpO2, oxygen saturation; HR, heart rate; HRR, heart rate reserve; ΔV′O2/HR, oxygen pulse; V′O2, oxygen uptake; AT, anaerobic threshold.
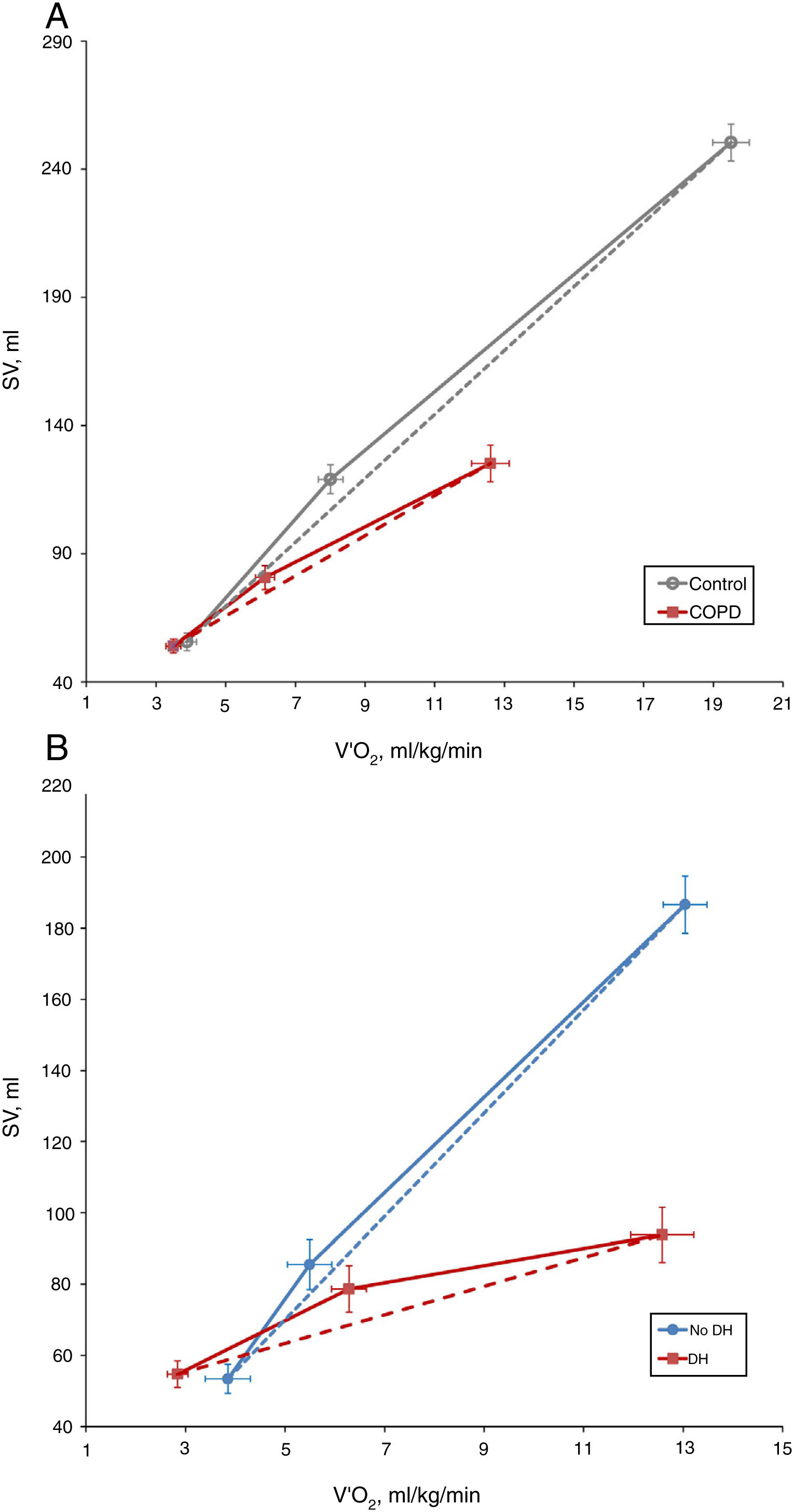
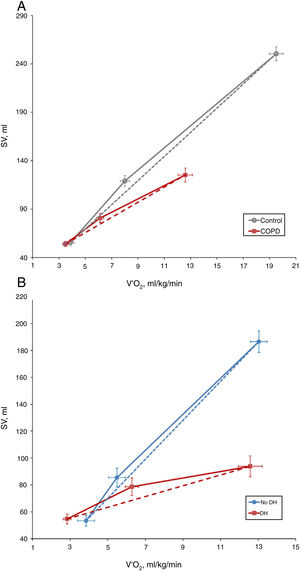
COPD patients showed a lower ΔSV/ΔVO2 slope than control subjects (7.91±8.66 vs. 12.55±8.54, P=0.035) (Fig. 1A). However, there were no differences between the two study groups in the exercise response of cardiac output (ΔCO/ΔVO2) or cardiac index (ΔIC/ΔVO2) (0.66±0.97 vs. 0.94±0.58, P=0.179; and 0.35±0.50 vs. 0.53±0.38, P=0.149, respectively). This discrepancy reflects the development of a compensatory mechanism at the expense of a greater increase in heart rate (HR), as demonstrated by the higher HR slope of COPD patients than control subjects (9.1±5.5 vs. 7.0±1.8 1/mL/kg, P=0.043).
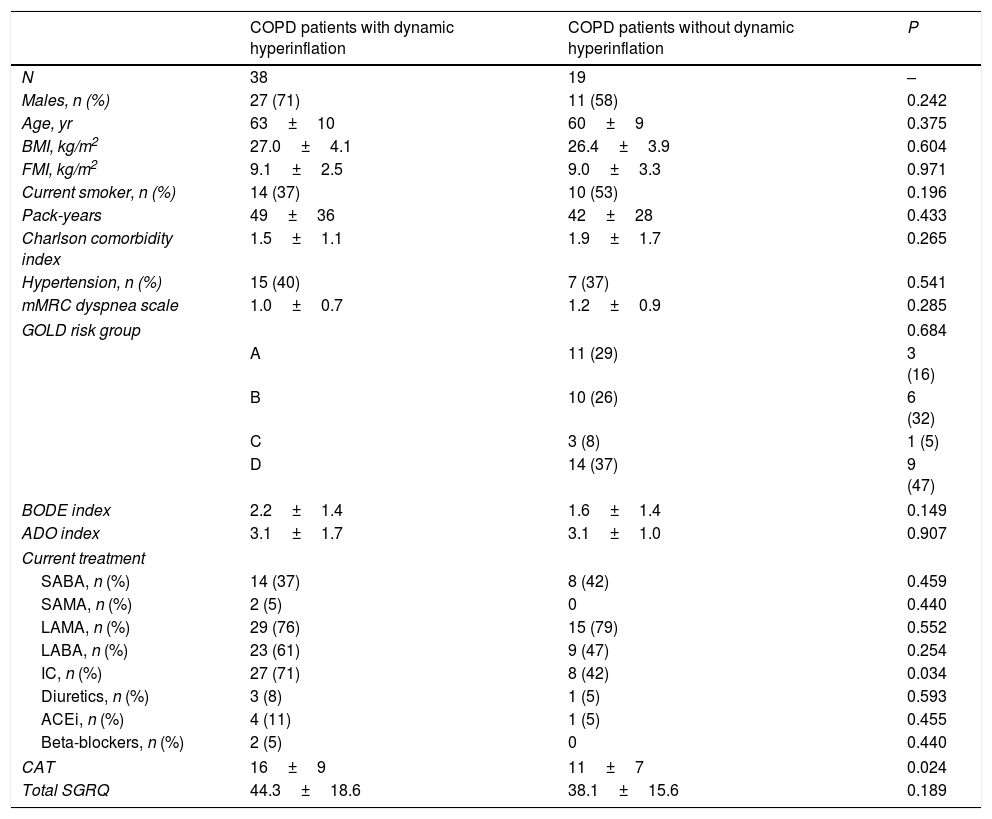
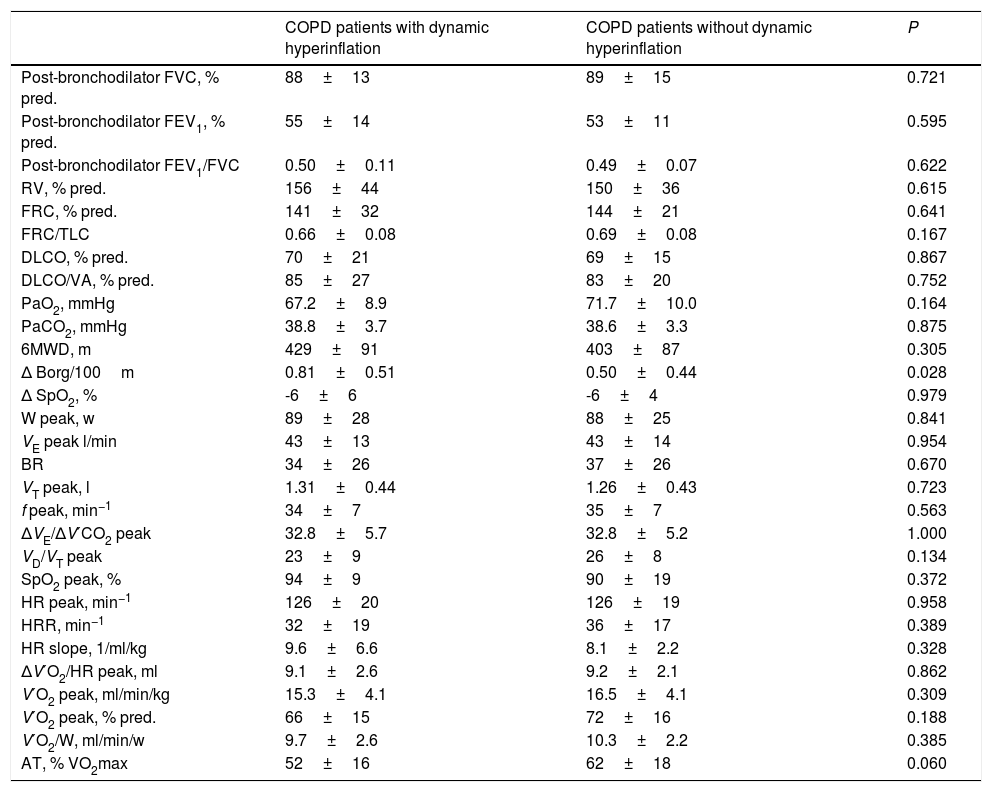
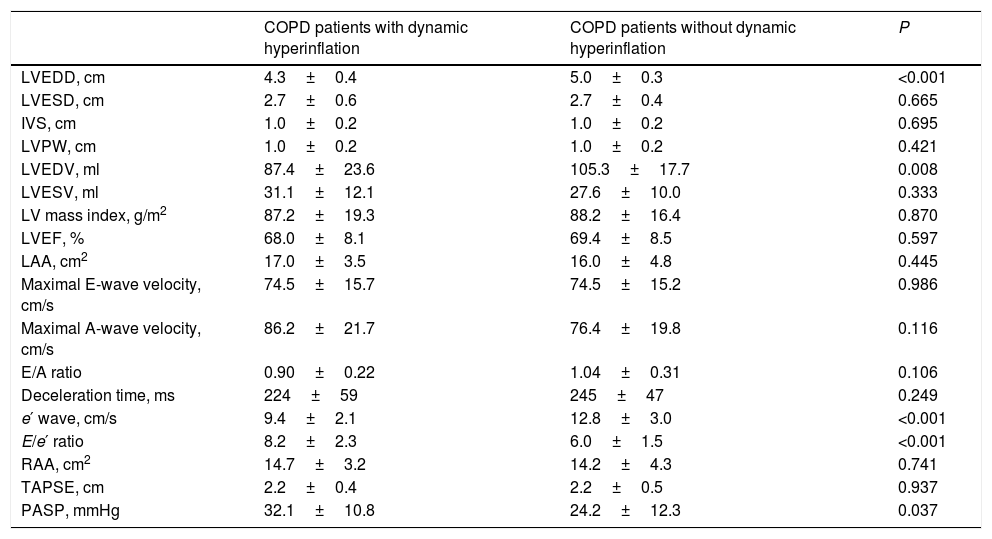
Specifically, 38 COPD patients developed DH, with a mean EELV increase of 0.62±0.59L. The comparison of COPD patients with or without DH did not identify differences in anthropometric characteristics, smoking, comorbidity, dyspnea level, GOLD risk group, BODE and ADO indices or current treatment (Table 2). The only difference was that COPD patients who presented DH had a worse CAT score than those without DH (16±9 vs. 11±7, P=0.024). Regarding exercise tolerance (Table 3) and lung parenchyma attenuation (Table S5), no significant differences were detected between COPD patients with or without DH. However, COPD patients who developed DH had a smaller LV end-diastolic diameter and volume, a lower early diastolic mitral wave, and a higher E/e′ ratio (Table 4), reflecting some impact on diastolic function. In fact, while diastolic function was normal in all COPD patients without DH, 23% of patients with DH had diastolic dysfunction grade I and 20% grade II (P=0.005). Furthermore, the left ventricle filling pressure was only elevated in one patient without DH compared to 17 with DH (P=0.006). In turn, the presence of DH was also accompanied by greater inflammatory tone (both systemic and in the airways), increased oxidative stress (reflected by a higher plasma concentration of 8-isoprostane), as well as increased plasma concentration of high-sensitivity cardiac troponin T (Table 5).
Comparison of COPD patients with and without dynamic hyperinflation.a
| COPD patients with dynamic hyperinflation | COPD patients without dynamic hyperinflation | P | |
|---|---|---|---|
| N | 38 | 19 | – |
| Males, n (%) | 27 (71) | 11 (58) | 0.242 |
| Age, yr | 63±10 | 60±9 | 0.375 |
| BMI, kg/m2 | 27.0±4.1 | 26.4±3.9 | 0.604 |
| FMI, kg/m2 | 9.1±2.5 | 9.0±3.3 | 0.971 |
| Current smoker, n (%) | 14 (37) | 10 (53) | 0.196 |
| Pack-years | 49±36 | 42±28 | 0.433 |
| Charlson comorbidity index | 1.5±1.1 | 1.9±1.7 | 0.265 |
| Hypertension, n (%) | 15 (40) | 7 (37) | 0.541 |
| mMRC dyspnea scale | 1.0±0.7 | 1.2±0.9 | 0.285 |
| GOLD risk group | 0.684 | ||
| A | 11 (29) | 3 (16) | |
| B | 10 (26) | 6 (32) | |
| C | 3 (8) | 1 (5) | |
| D | 14 (37) | 9 (47) | |
| BODE index | 2.2±1.4 | 1.6±1.4 | 0.149 |
| ADO index | 3.1±1.7 | 3.1±1.0 | 0.907 |
| Current treatment | |||
| SABA, n (%) | 14 (37) | 8 (42) | 0.459 |
| SAMA, n (%) | 2 (5) | 0 | 0.440 |
| LAMA, n (%) | 29 (76) | 15 (79) | 0.552 |
| LABA, n (%) | 23 (61) | 9 (47) | 0.254 |
| IC, n (%) | 27 (71) | 8 (42) | 0.034 |
| Diuretics, n (%) | 3 (8) | 1 (5) | 0.593 |
| ACEi, n (%) | 4 (11) | 1 (5) | 0.455 |
| Beta-blockers, n (%) | 2 (5) | 0 | 0.440 |
| CAT | 16±9 | 11±7 | 0.024 |
| Total SGRQ | 44.3±18.6 | 38.1±15.6 | 0.189 |
Definition of abbreviations: BMI, body mass index; FMI, fat mass index; mMRC, modified Medical Research Council; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SABA, short-acting beta agonists; SAMA, short-acting muscarinic antagonist; LAMA, long-acting muscarinic antagonist; LABA, long-acting beta agonists; IC, inhaled corticosteroids; ACEi, angiotensin-converting-enzyme inhibitors; CAT, COPD assessment test; SGRQ, St George Respiratory Questionnaire.
Comparison of functional characteristics and exercise response in COPD patients with and without dynamic hyperinflation.a
| COPD patients with dynamic hyperinflation | COPD patients without dynamic hyperinflation | P | |
|---|---|---|---|
| Post-bronchodilator FVC, % pred. | 88±13 | 89±15 | 0.721 |
| Post-bronchodilator FEV1, % pred. | 55±14 | 53±11 | 0.595 |
| Post-bronchodilator FEV1/FVC | 0.50±0.11 | 0.49±0.07 | 0.622 |
| RV, % pred. | 156±44 | 150±36 | 0.615 |
| FRC, % pred. | 141±32 | 144±21 | 0.641 |
| FRC/TLC | 0.66±0.08 | 0.69±0.08 | 0.167 |
| DLCO, % pred. | 70±21 | 69±15 | 0.867 |
| DLCO/VA, % pred. | 85±27 | 83±20 | 0.752 |
| PaO2, mmHg | 67.2±8.9 | 71.7±10.0 | 0.164 |
| PaCO2, mmHg | 38.8±3.7 | 38.6±3.3 | 0.875 |
| 6MWD, m | 429±91 | 403±87 | 0.305 |
| Δ Borg/100m | 0.81±0.51 | 0.50±0.44 | 0.028 |
| Δ SpO2, % | -6±6 | -6±4 | 0.979 |
| W peak, w | 89±28 | 88±25 | 0.841 |
| VE peak l/min | 43±13 | 43±14 | 0.954 |
| BR | 34±26 | 37±26 | 0.670 |
| VT peak, l | 1.31±0.44 | 1.26±0.43 | 0.723 |
| f peak, min−1 | 34±7 | 35±7 | 0.563 |
| ΔVE/ΔV′CO2 peak | 32.8±5.7 | 32.8±5.2 | 1.000 |
| VD/VT peak | 23±9 | 26±8 | 0.134 |
| SpO2 peak, % | 94±9 | 90±19 | 0.372 |
| HR peak, min−1 | 126±20 | 126±19 | 0.958 |
| HRR, min−1 | 32±19 | 36±17 | 0.389 |
| HR slope, 1/ml/kg | 9.6±6.6 | 8.1±2.2 | 0.328 |
| ΔV′O2/HR peak, ml | 9.1±2.6 | 9.2±2.1 | 0.862 |
| V′O2 peak, ml/min/kg | 15.3±4.1 | 16.5±4.1 | 0.309 |
| V′O2 peak, % pred. | 66±15 | 72±16 | 0.188 |
| V′O2/W, ml/min/w | 9.7±2.6 | 10.3±2.2 | 0.385 |
| AT, % VO2max | 52±16 | 62±18 | 0.060 |
Definition of abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FRC, functional residual capacity; TLC, total lung capacity; RV, residual volume; DLCO, carbon monoxide diffusing capacity; VA, alveolar volume; PaO2, arterial oxygen pressure; PaCO2, carbon dioxide arterial pressure; 6MWD, 6-minute walk distance; W, work intensity; VE, minute ventilation; BR, breathing reserve; VT, tidal volume; f, respiratory frequency; ΔVE/ΔV′CO2, ventilatory equivalent for carbon dioxide; VD/VT, ratio of physiologic dead space to tidal volume; SpO2, oxygen saturation; HR, heart rate; HRR, heart rate reserve; ΔV′O2/HR, oxygen pulse; V′O2, oxygen uptake; AT, anaerobic threshold.
Comparison of echocardiographic parameters between COPD patients with or without dynamic hyperinflation.a
| COPD patients with dynamic hyperinflation | COPD patients without dynamic hyperinflation | P | |
|---|---|---|---|
| LVEDD, cm | 4.3±0.4 | 5.0±0.3 | <0.001 |
| LVESD, cm | 2.7±0.6 | 2.7±0.4 | 0.665 |
| IVS, cm | 1.0±0.2 | 1.0±0.2 | 0.695 |
| LVPW, cm | 1.0±0.2 | 1.0±0.2 | 0.421 |
| LVEDV, ml | 87.4±23.6 | 105.3±17.7 | 0.008 |
| LVESV, ml | 31.1±12.1 | 27.6±10.0 | 0.333 |
| LV mass index, g/m2 | 87.2±19.3 | 88.2±16.4 | 0.870 |
| LVEF, % | 68.0±8.1 | 69.4±8.5 | 0.597 |
| LAA, cm2 | 17.0±3.5 | 16.0±4.8 | 0.445 |
| Maximal E-wave velocity, cm/s | 74.5±15.7 | 74.5±15.2 | 0.986 |
| Maximal A-wave velocity, cm/s | 86.2±21.7 | 76.4±19.8 | 0.116 |
| E/A ratio | 0.90±0.22 | 1.04±0.31 | 0.106 |
| Deceleration time, ms | 224±59 | 245±47 | 0.249 |
| e′ wave, cm/s | 9.4±2.1 | 12.8±3.0 | <0.001 |
| E/e′ ratio | 8.2±2.3 | 6.0±1.5 | <0.001 |
| RAA, cm2 | 14.7±3.2 | 14.2±4.3 | 0.741 |
| TAPSE, cm | 2.2±0.4 | 2.2±0.5 | 0.937 |
| PASP, mmHg | 32.1±10.8 | 24.2±12.3 | 0.037 |
Definition of abbreviations: LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end-systolic diameter; IVS, Interventricular septum; LVPW, posterior wall of left ventricle; LVEDV, left ventricle end-diastolic volume; LVESV, left ventricle end-systolic volume; LV, left ventricle; LVEF, Left ventricle ejection fraction; LAA, left atrial area; e′, early diastolic mitral wave; RAA, right atrial area; TAPSE, tricuspid annular plane excursion; PASP, pulmonary artery systolic pressure.
Comparison of biomarker levels between COPD patients with or without dynamic hyperinflation.a
| COPD patients with dynamic hyperinflation | COPD patients without dynamic hyperinflation | P† | |
|---|---|---|---|
| IL17A, pg/mL | 3.92 (2.76–5.86) | 3.72 (2.16–4.89) | 0.388 |
| IL-1β, pg/mL | 1.27 (0.92–1.52) | 0.49 (0.35–0.61) | <0.001 |
| IL-6, pg/mL | 1.98 (1.55–2.78) | 0.67 (0.43–1.10) | <0.001 |
| IL-8, pg/mL | 3.56 (3.11–4.64) | 1.48 (1.34–2.03) | <0.001 |
| MIP-1α, pg/mL | 10.3 (8.2–13.0) | 9.8 (5.7–12.0) | 0.563 |
| TNFα, pg/mL | 4.4 (3.1–5.9) | 4.0 (3.1–5.1) | 0.855 |
| Homocysteine, μmoL/L | 12.5 (11.1–16.4) | 11.8 (9.9–13.8) | 0.123 |
| NT-proBNP, pg/mL | 73.6 (26.4–189.5) | 76.3 (54.3–216.2) | 0.330 |
| CRP, mg/L | 3.1 (2.9–5.5) | 2.9 (2.9–5.9) | 0.703 |
| hs-ctnT, ng/L | 9.7 (5.3–14.1) | 3.2 (3.0–5.2) | <0.001 |
| Galectine-3, ng/mL | 3.6 (3.0–4.5) | 4.4 (3.5–4.8) | 0.188 |
| 8-isoprostane, pg/mL | 633±112 | 438±135 | <0.001 |
| Glutathione peroxidases (GPX), mg/mL | 5.5 (1.9–18.8) | 1.4 (0.2–4.1) | 0.006 |
| N-terminal type III procollagen (PIIINP) | 4552 (3724–5769) | 4519 (3973–5164) | 0.822 |
| IL-1β in EBC, pg/mL | 0.24 (0.22–0.41) | 0.19 (0.17–0.21) | <0.001 |
| IL-6 in EBC, pg/mL | 0.05 (0.04–0.11) | 0.03 (0.03–0.04) | <0.001 |
| IL-8 in EBC, pg/mL | 0.12 (0.06–0.18) | 0.06 (0.05–0.06) | <0.001 |
| TNFα in EBC, pg/mL | 0.27 (0.26–0.30) | 0.26 (0.24–0.31) | 0.251 |
Definition of abbreviations: IL, interleukin; MIP, Macrophage inflammatory protein 1; TNF, tumor necrosis factor; NT-proBNP, N-terminal pro-B-type natriuretic peptide; CRP, C-reactive protein; hs-ctnT, high-sensitive cardiac troponin T; EBC, exhaled breath condensate.
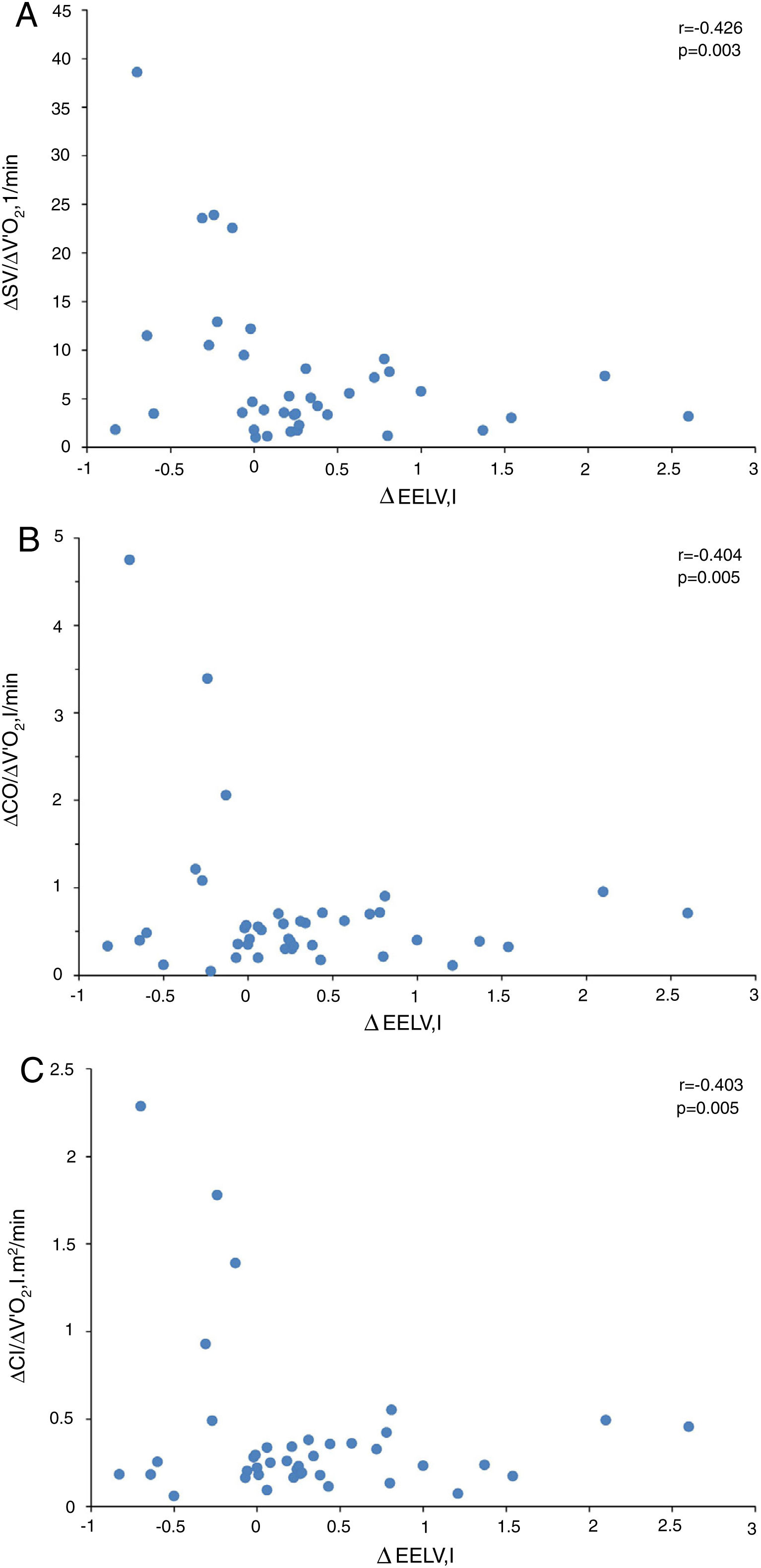
In COPD patients, the EELV increase was related to the inspiratory-expiratory difference of the P15 (r=−0.458, P=0.001), early diastolic mitral wave (r=0.339, P=0.015), E/e′ ratio (r=0.339, P=0.015), right atrial area (r=−0.394, P=0.042) and pulmonary artery systolic pressure (r=0.422, P=0.008), as well as plasma concentrations of IL-1β (r=0.320, P=0.016), 8-isoprostane (r=0.323, P=0.021) and hs-ctnT (r=0.468, P=0.001) and with EBC concentrations of IL-6 (r=0.328, P=0.015) and IL-8 (r=0.301, P=0.027) (Figs. S1–S4).
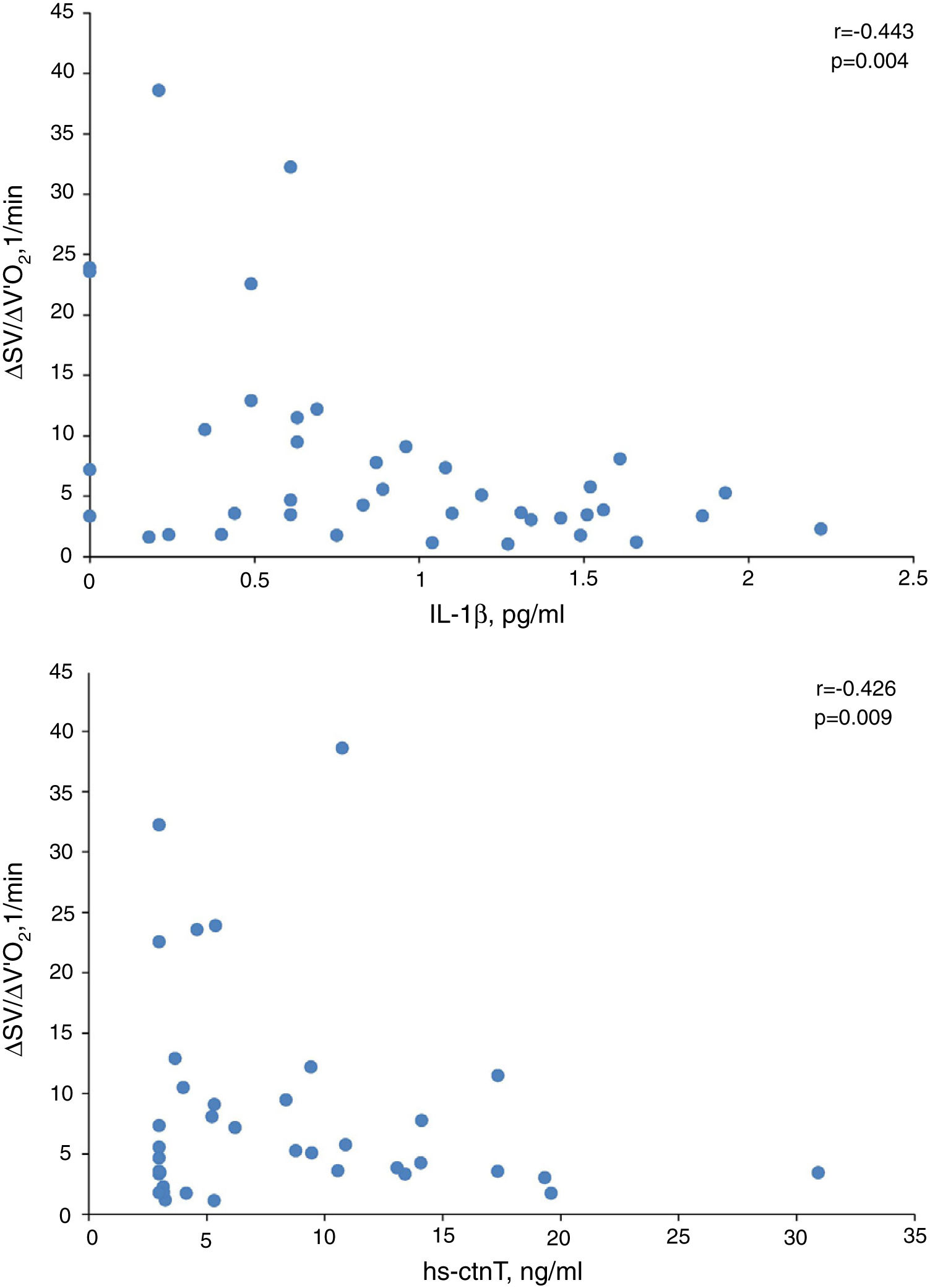
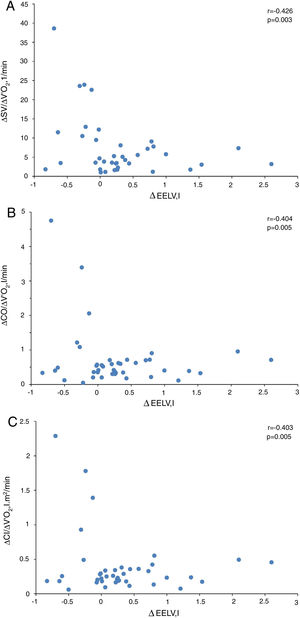
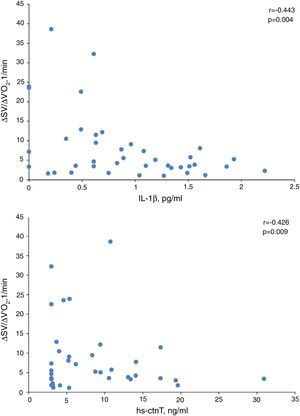
In presence of dynamic hyperinflation, COPD patients experienced a decrease in the cardiac response to exercise, with lower values of ΔSV/ΔVO2 (4.14±2.36 vs. 14.19±11.49, P=0.005) (Fig. 1B)-, ΔCO/ΔVO2 (0.36±0.48 vs. 1.18±1.39, P=0.035) and ΔCI/ΔVO2 (0.20±0.24 vs. 0.65±0.72, P=0.029) than COPD patients without DH. Overall, the increase in EELV was inversely related to SV, CO and CI response to exercise (Fig. 2). In addition, the ΔSV/ΔVO2 of COPD patients was also directly related to their LVEDD (r=0.345, P=0.037) and inversely related to plasma levels of IL-1β (r=−0.443, P=0.004), IL-8 (r=−0.373, P=0.025), hs-ctnT (r=−0.426, P=0.009) and 8-isoprostane (r=−0.379, P=0.023), as well as EBC concentrations of IL-6 (r=−0.403, P=0.011) and IL-8 (r=−0.319, P=0.048) (Fig. 3 and S5).
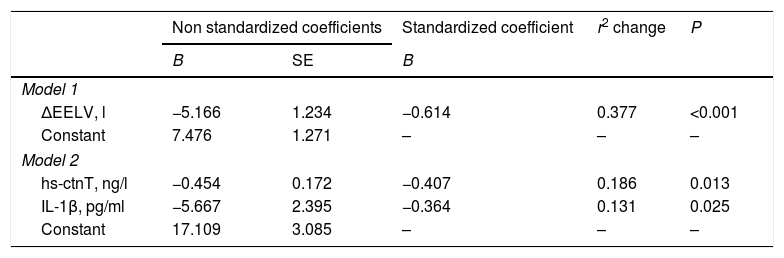
Table 6 shows the multiple linear regression models of the selected parameters as independent predictors of ΔSV/ΔVO2 in COPD patients. When all the variables significantly related to ΔSV/ΔVO2 were included, the only independent predictor was the EELV increase, reflecting the major contribution of DH to impairment of cardiac response to exercise in COPD patients. However, when this parameter was excluded (model 2), plasma levels of IL-1β and hs-ctnT were retained as independent predictors of the SV response to exercise (Fig. 3), which demonstrates a relevant contribution of systemic inflammation and the existence of a certain degree of myocardial damage.
Determinants of stroke volume response to exercise in COPD patients in multivariate linear regression analysis models.a
| Non standardized coefficients | Standardized coefficient | r2 change | P | ||
|---|---|---|---|---|---|
| B | SE | B | |||
| Model 1 | |||||
| ΔEELV, l | −5.166 | 1.234 | −0.614 | 0.377 | <0.001 |
| Constant | 7.476 | 1.271 | – | – | – |
| Model 2 | |||||
| hs-ctnT, ng/l | −0.454 | 0.172 | −0.407 | 0.186 | 0.013 |
| IL-1β, pg/ml | −5.667 | 2.395 | −0.364 | 0.131 | 0.025 |
| Constant | 17.109 | 3.085 | – | – | – |
Definition of abbreviations: IL, interleukin; hs-ctnT, high-sensitive cardiac troponin T; EELV, end-expiratory lung volume.
Our results show that patients with COPD experience a smaller increase in SV during exercise than control subjects, and that this reduction in cardiac response to exercise is fundamentally associated with the development of DH.
While patients with COPD demonstrated a lower increase in SV during exercise compared to control subjects, we identified no differences between the two groups in the response of CO or CI. This suggests the existence of a compensatory effect on heart rate that, as previously described in classic hemodynamic studies,12,28 increases more notably in patients with COPD than in control subjects. In any case, our results do not rule out a reduction in CO response at maximum exercise, since the determinations were done at lower load intensities. In fact, other authors have described that, in patients with COPD, CO increases up to a load corresponding to 50% of peak work capacity, while at higher intensities its increase in relation to VO2 is attenuated.29
Regarding patients with COPD, our results demonstrate that those who develop DH have a marked reduction in cardiac response to exercise, both in terms of SV and CO. Previously, it had been suggested that dynamic hyperinflation could compromise cardiac response to exercise in patients with COPD, through indirect indicators such as oxygen pulse.13 However, to our knowledge, our results are the first using specific measurements, instead of estimating from surrogate parameters, to confirm the depressant effect of DH on the increase in SV and CO during exercise in patients with COPD. Unlike other authors, in our case, we have not identified a relationship between the severity of airflow limitation or static hyperinflation and cardiac response to exercise. In eight COPD patients with no known cardiopathy in whom CO was measured by thoracic electrical bioimpedance, those with severe or very severe airflow limitation were reported to have a slower CO response to exercise than patients with mild-moderate involvement.30 It is possible that the difference in the degree of airflow limitation with respect to our patients justifies this apparent discrepancy. In turn, Vassaux et al.10 have reported that severe static hyperinflation is associated with a lower peak and baseline oxygen pulse, a circumstance that is also not confirmed in our patients using objective cardiac function measurements. In any case, it should also be taken into account that the prevalence of severe static hyperinflation in our sample has been relatively low (14%).
From a theoretical point of view, the potential deleterious effect of DH on the LV response to exercise has been mainly attributed to a decrease in preload, due to the reduction of filling pressure and LV compliance, compression of the cardiac fossa and increased compliance of the extra-alveolar vessels.31 However, our results do not demonstrate the existence of a relationship between the SV response to exercise and the size of the cardiac chambers or the baseline diastolic function of patients with COPD. These findings concur with a simulation study of DH in healthy subjects subjected to expiratory loads,32 which found that the reduction of SV only seems to be due to a decrease in diastolic filling of the LV when DH is very mild, while at a greater intensity of DH the reduction in SV seems to be due to a mechanism of direct ventricular interaction.32 In this context, it is very attractive to pose whether DH could have a direct effect on the cardiac chambers, either by direct compression or mediated by other intermediate pathways. As already mentioned, the first evidence of the impact of DH on myocardial contractility was provided by Tzani et al.,13 who demonstrated that the increase in EELV of patients with COPD is related to a lower increase in the oxygen pulse but also with a lower DP reserve. It is important to consider that the DP reserve is an indicator of the maximum performance of the LV, which reflects the myocardial oxygen consumption during exercise, depending on the contractile state of the heart.33 In fact, classical studies have shown that the consumption of oxygen by the myocardium during exercise can be reliably estimated based on the DP value.34 Therefore, the lower cardiac response to exercise seen in patients who develop DH could also be a consequence of impaired intrinsic LV contractility.
Thus, it is very interesting that when the EELV is eliminated from the multiple linear regression models, which nullifies the effect of all the parameters related to DH, the baseline levels of IL-1β and high-sensitivity cardiac troponin T are independent predictors of SV response to exercise, reflecting a potential contribution of systemic inflammation and myocardial damage. In fact, IL-1β may be an essential mediator in the pathogenesis of heart failure by suppressing cardiac contractility, promoting myocardial hypertrophy and inducing cardiomyocyte apoptosis.35 Several in vivo and in vitro studies reinforce the potential effect of IL-1β on LV contractility. In in vitro models, prolonged exposure to IL-1β induces a reversible alteration in the excitation-contraction coupling of cultured cardiomyocytes.36 Significant murine models have shown that the administration of IL-1β induces reversible LV contractile dysfunction,37 while its blockage improves LV function and restores contractility.38 Thus, IL-1β has been found to depress cardiac function through the NO-dependent35 and NO-independent pathways and inhibit β-adrenergic agonist-mediated increase in cardiac myocyte contractility and cAMP accumulation.39 Moreover, IL-1β, alone or in combination with IFN-γ and TNF-α, induces cardiomyocyte apoptosis, associated with the activation of Bak and Bcl-xL through pathways involving nitric oxide (NO).40 Although the significance of IL-1β mediated suppression of function in most cardiac pathologic conditions remains poorly defined, evidence suggests that IL-1β is an essential mediator in some clinical situations, such as sepsis-induced contractile dysfunction.35
As for hs-cTnT, its increase has been described in many cardiac and extracardiac conditions other than ischemic cardiomyopathy.41 Given that our patients presented no evidence of sepsis, heart failure, tachyarrhythmia, hypo or hypertension, stroke, respiratory failure, renal failure, severe anemia, pulmonary embolism, seizures or aortic stenosis, it is plausible that the increased levels of hs-cTnT are related with a low-grade inflammatory state,41 as reflected in the relationship between serum concentrations of IL-1β and hs-cTnT recently described in patients with heart failure.42 In this case, the identification of higher hs-cTnT values in COPD patients who develop DH and its relationship with the SV response to exercise constitutes another finding that could suggest a certain degree of myocardial stress. In fact, in previous studies conducted both in patients with stable chronic heart failure as with newly diagnosed hypertension, serum hs-cTnT concentrations were related to LV ejection fraction43–45 and it is generally accepted that hs-cTnT elevation is a reliable biomarker of cardiomyocyte microinjury and hemodynamic stress.46
In our opinion, the main strengths of the present study lie in the strict selection and characterization of patients with COPD without previous evidence of cardiac involvement and in the specific measurement of SV and CO. Likewise, we also recognize several limitations. First, this is a single-center study conducted with a limited number of subjects, although sufficient to detect differences in cardiac response to exercise according to the previously estimated sample size. Second, the characteristics and duration of the rebreathing procedure make it difficult to obtain consistent SV and CO determinations at peak exercise, so we only assess the cardiac response at submaximal exercise. Third, it was not possible to simultaneously measure SV and CO with stress echocardiography, so there is no evaluation of the diastolic function of LV during exercise. Fourth, the spectrum of patients evaluated corresponds mainly to patients with moderate airflow limitation, so the results may not be extrapolated to very severe forms of disease.
In conclusion, our study demonstrates that, in COPD patients with no known heart disease, DH is associated with a lower increase in SV and CO during exercise. This depression of the cardiac response to exercise is unrelated to LV diastolic dysfunction yet does maintain an independent association with biomarkers of systemic inflammation and myocardial stress. Therefore, it could be speculated that DH also has an effect on LV contractility mediated by inflammation.
FundingThis study was supported by grants fromInstituto de Salud Carlos III-Fondos FEDERPI10-02089 and PI16/00201 to F. García-Río.
Conflict of interestNone declared.