Non-invasive respiratory support (NIRS) in adult, pediatric, and neonatal patients with acute respiratory failure (ARF) comprises two treatment modalities, non-invasive mechanical ventilation (NIMV) and high-flow nasal cannula (HFNC) therapy. However, experts from different specialties disagree on the benefit of these techniques in different clinical settings. The objective of this consensus was to develop a series of good clinical practice recommendations for the application of non-invasive support in patients with ARF, endorsed by all scientific societies involved in the management of adult and pediatric/neonatal patients with ARF.
To this end, the different societies involved were contacted, and they in turn appointed a group of 26 professionals with sufficient experience in the use of these techniques. Three face-to-face meetings were held to agree on recommendations (up to a total of 71) based on a literature review and the latest evidence associated with 3 categories: indications, monitoring and follow-up of NIRS. Finally, the experts from each scientific society involved voted telematically on each of the recommendations. To classify the degree of agreement, an analog classification system was chosen that was easy and intuitive to use and that clearly stated whether the each NIRS intervention should be applied, could be applied, or should not be applied.
El soporte respiratorio no invasivo (SRNI) comprende 2 modalidades de tratamiento, la ventilación mecánica no invasiva (VMNI) y la terapia de alto flujo con cánulas nasales (TAFCN) que se aplican en pacientes adultos, pediátricos y neonatales con insuficiencia respiratoria aguda (IRA). Sin embargo, el grado de acuerdo entre las distintas especialidades sobre el beneficio de estas técnicas en diferentes escenarios clínicos es controvertido. El objetivo del presente consenso fue elaborar una serie de recomendaciones de buena práctica clínica para la aplicación de soporte no invasivo en pacientes con IRA, avaladas por todas las sociedades científicas involucradas en el manejo del paciente adulto y pediátrico/neonatal con IRA.
Para ello se contactó con las diferentes sociedades implicadas, quienes designaron a su vez a un grupo de 26 profesionales con suficiente experiencia en su aplicación. Se realizaron 3 reuniones presenciales para consensuar las recomendaciones (hasta un total de 71) fundamentadas en la revisión de la literatura y en la actualización de la evidencia disponible en relación con 3 categorías: indicaciones, monitorización y seguimiento del SRNI. Finalmente, se procedió a votación telemática de cada una de las recomendaciones, por parte de los expertos de cada sociedad científica implicada. Para la clasificación del grado de acuerdo se optó por un sistema analógico de clasificación fácil e intuitivo de usar, y que expresara con claridad si el procedimiento relacionado con el SRNI debía hacerse, podía hacerse o no debía hacerse.
Non-invasive respiratory support (NIRS) comprises 2 treatment modalities, non-invasive mechanical ventilation (NIMV) and high-flow nasal cannula (HFNC) therapy, and is used in adult, pediatric, and neonatal patients with acute respiratory failure (ARF). Advances in scientific knowledge on its application in various severe diseases have led to its widespread use. However, there is some controversy among specialists from different areas on the benefit of these techniques in different clinical scenarios, since no consensus document is available on the use of non-invasive support in ARF, and much less one that covers all age groups. This situation may compromise patient safety and quality of care during the use of these techniques.
The objective of this consensus document was to develop a series of good clinical practice recommendations for the application of non-invasive support in patients with ARF, endorsed by all scientific societies involved in the management of adult and pediatric/neonatal patients. The respective societies designated experts in the field who would best represent their area in the working group. To support the consensus, we performed a literature review (overview), updated the available evidence in 3 categories (indications, monitoring, and follow-up), and developed recommendations for clinical actions based on the consensus of professionals where there was evidence of sufficient quality and high concordance.
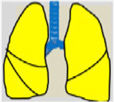
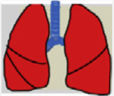
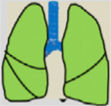
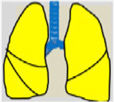
Consensus recommendations (clinical practice suggestions) were based where possible on quality evidence derived from available published data. Otherwise, the tacit consensus of the members of the working group was accepted. An easy and intuitive analog system was used to classify the degree of agreement.1 Thus, a green symbol (lung) indicates a consensus recommendation of “should be used”: a treatment or procedure indicated on the basis of at least one randomized clinical trial or benefit or efficacy supported by solid observational evidence. A “yellow lung” indicates general agreement and/or scientific evidence that it “may be used”. This statement or the usefulness/efficacy of a treatment or procedure is based on clinical trials in a small number of patients or outcomes that may not be widely applicable to all patients with such characteristics. Finally, management strategies for which there is scientific evidence of potential harm or malpractice and which, therefore, should not be promoted (“do not use”) are indicated by a “red lung” (Table 1). To define the degree of agreement, participants voted telematically once the key points had been defined. Voting groups were defined by the patient's age group (adult, pediatric, neonatal), in such a way that each specialist could only vote in the group to which they had been previously assigned based on their experience.
Scientific Rationale for Definitions Based on the “lung color” Classification of Clinical Practice Suggestions.
| Definitions Related to a Treatment or Procedure | Consensus Statement | Symbol |
|---|---|---|
| Scientific evidence that a treatment or procedure is beneficial and effective. Requires at least one randomized clinical trial or is supported by strong observational evidence and authors’ consensus (as indicated by an asterisk) | Should be used | |
| General agreement and/or scientific evidence favor the usefulness/efficacy of a treatment or procedure. May be supported by randomized clinical trials based on a small number of patients or not widely applicable | May be used | |
| Scientific evidence or general agreement not to use or recommend a treatment or procedure | Must not be used |
This summary includes the main recommendations and key points in each of the 3 sections mentioned above. These recommendations are also listed in Tables 2–8, which show the consensus percentage achieved by that option: a consensus of 50% or more members was required to make the corresponding recommendation or suggestion. If 2 options achieved the same number of votes (may or should be used), both were reflected in the document. The full document is also available as an online supplement.
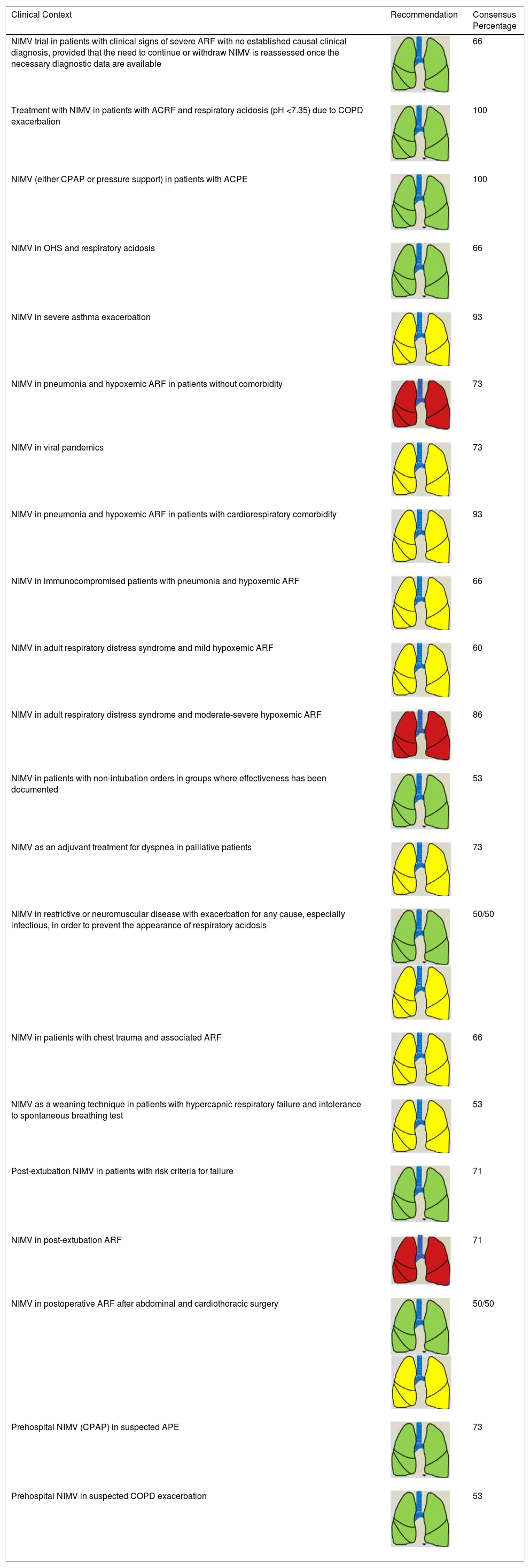
Consensus Recommendations for NIRS Indications (NIMV) in Adult Patients.
| Clinical Context | Recommendation | Consensus Percentage |
|---|---|---|
| NIMV trial in patients with clinical signs of severe ARF with no established causal clinical diagnosis, provided that the need to continue or withdraw NIMV is reassessed once the necessary diagnostic data are available | 66 | |
| Treatment with NIMV in patients with ACRF and respiratory acidosis (pH <7.35) due to COPD exacerbation | 100 | |
| NIMV (either CPAP or pressure support) in patients with ACPE | 100 | |
| NIMV in OHS and respiratory acidosis | 66 | |
| NIMV in severe asthma exacerbation | 93 | |
| NIMV in pneumonia and hypoxemic ARF in patients without comorbidity | 73 | |
| NIMV in viral pandemics | 73 | |
| NIMV in pneumonia and hypoxemic ARF in patients with cardiorespiratory comorbidity | 93 | |
| NIMV in immunocompromised patients with pneumonia and hypoxemic ARF | 66 | |
| NIMV in adult respiratory distress syndrome and mild hypoxemic ARF | 60 | |
| NIMV in adult respiratory distress syndrome and moderate-severe hypoxemic ARF | 86 | |
| NIMV in patients with non-intubation orders in groups where effectiveness has been documented | 53 | |
| NIMV as an adjuvant treatment for dyspnea in palliative patients | 73 | |
| NIMV in restrictive or neuromuscular disease with exacerbation for any cause, especially infectious, in order to prevent the appearance of respiratory acidosis | 50/50 | |
| NIMV in patients with chest trauma and associated ARF | 66 | |
| NIMV as a weaning technique in patients with hypercapnic respiratory failure and intolerance to spontaneous breathing test | 53 | |
| Post-extubation NIMV in patients with risk criteria for failure | 71 | |
| NIMV in post-extubation ARF | 71 | |
| NIMV in postoperative ARF after abdominal and cardiothoracic surgery | 50/50 | |
| Prehospital NIMV (CPAP) in suspected APE | 73 | |
| Prehospital NIMV in suspected COPD exacerbation | 53 |
ACRF: acute-on-chronic respiratory failure; ACPE: acute cardiogenic pulmonary edema; ARF: acute respiratory failure; COPD: chronic obstructive pulmonary disease; CPAP: continuous positive pressure; NIMV: non-invasive mechanical ventilation; NIRS: non-invasive respiratory support.
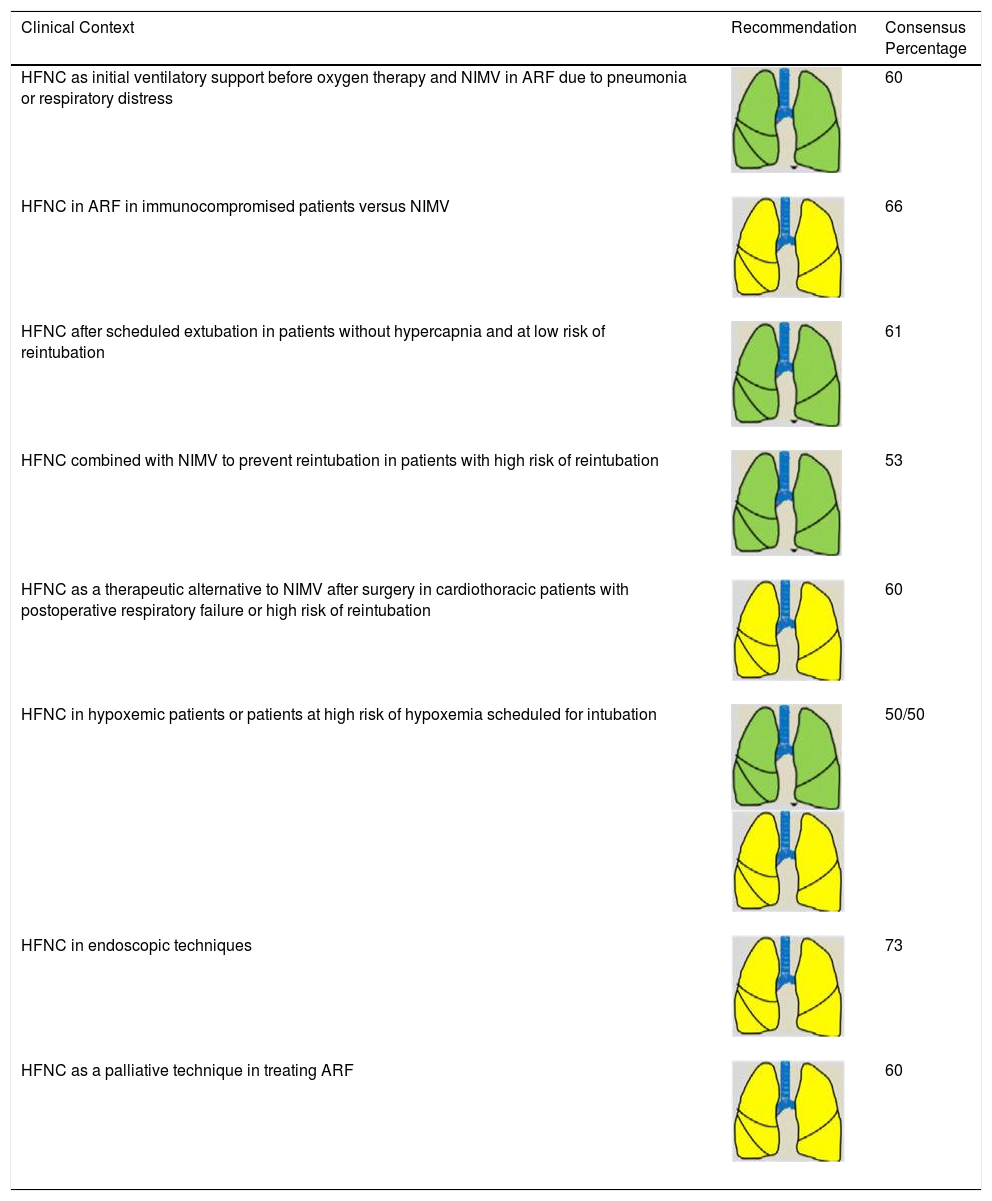
Consensus Recommendations for NIRS Indications (HFNC) in Adult Patients.
| Clinical Context | Recommendation | Consensus Percentage |
|---|---|---|
| HFNC as initial ventilatory support before oxygen therapy and NIMV in ARF due to pneumonia or respiratory distress | 60 | |
| HFNC in ARF in immunocompromised patients versus NIMV | 66 | |
| HFNC after scheduled extubation in patients without hypercapnia and at low risk of reintubation | 61 | |
| HFNC combined with NIMV to prevent reintubation in patients with high risk of reintubation | 53 | |
| HFNC as a therapeutic alternative to NIMV after surgery in cardiothoracic patients with postoperative respiratory failure or high risk of reintubation | 60 | |
| HFNC in hypoxemic patients or patients at high risk of hypoxemia scheduled for intubation | 50/50 | |
| HFNC in endoscopic techniques | 73 | |
| HFNC as a palliative technique in treating ARF | 60 |
ARF: acute respiratory failure; HFNC: high-flow nasal cannula therapy; NIMV: non-invasive mechanical ventilation; NIRS: non-invasive respiratory support.
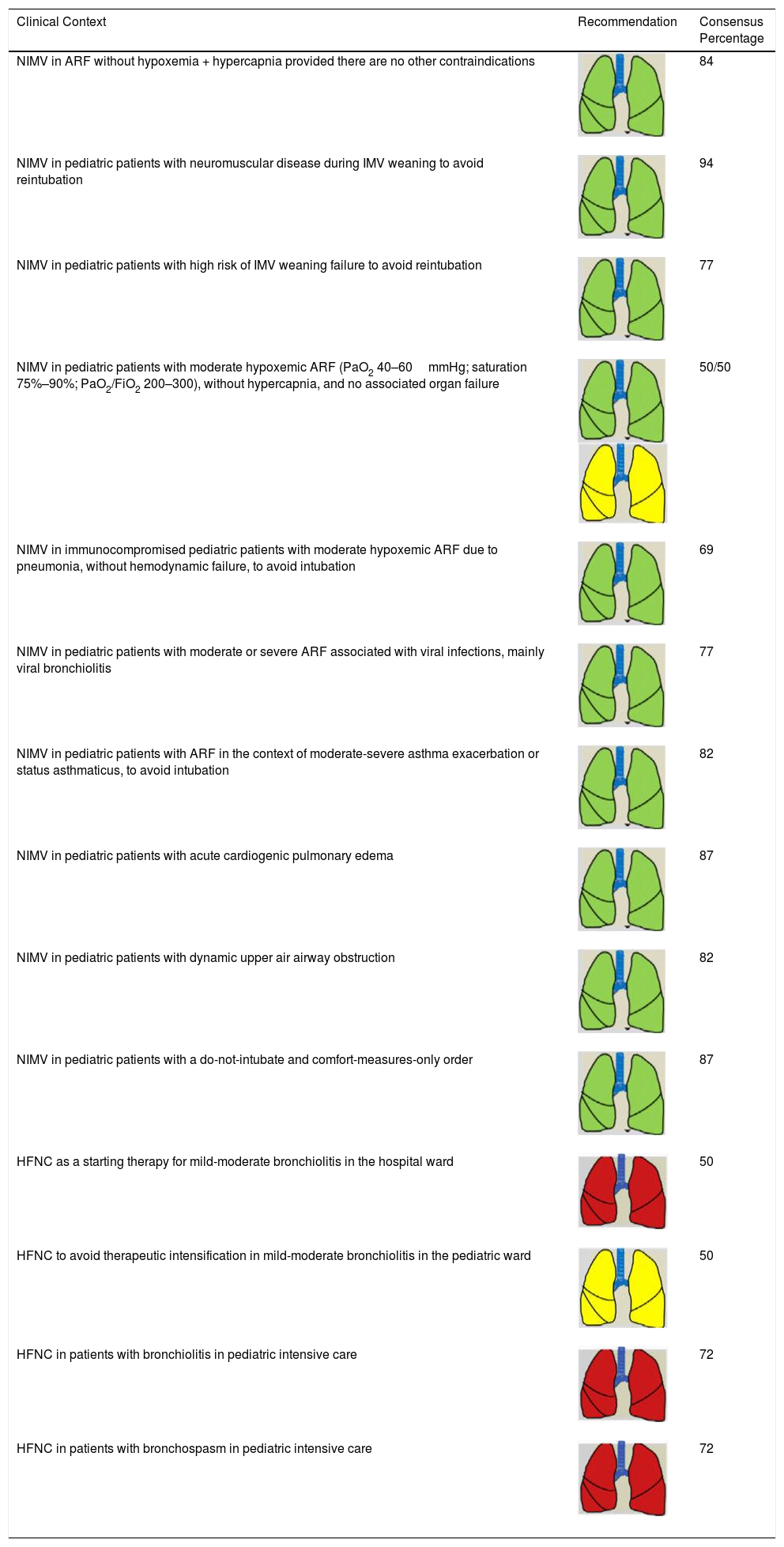
Consensus Recommendations for Indicating NIRS in Pediatric Patients.
| Clinical Context | Recommendation | Consensus Percentage |
|---|---|---|
| NIMV in ARF without hypoxemia + hypercapnia provided there are no other contraindications | 84 | |
| NIMV in pediatric patients with neuromuscular disease during IMV weaning to avoid reintubation | 94 | |
| NIMV in pediatric patients with high risk of IMV weaning failure to avoid reintubation | 77 | |
| NIMV in pediatric patients with moderate hypoxemic ARF (PaO2 40–60mmHg; saturation 75%–90%; PaO2/FiO2 200–300), without hypercapnia, and no associated organ failure | 50/50 | |
| NIMV in immunocompromised pediatric patients with moderate hypoxemic ARF due to pneumonia, without hemodynamic failure, to avoid intubation | 69 | |
| NIMV in pediatric patients with moderate or severe ARF associated with viral infections, mainly viral bronchiolitis | 77 | |
| NIMV in pediatric patients with ARF in the context of moderate-severe asthma exacerbation or status asthmaticus, to avoid intubation | 82 | |
| NIMV in pediatric patients with acute cardiogenic pulmonary edema | 87 | |
| NIMV in pediatric patients with dynamic upper air airway obstruction | 82 | |
| NIMV in pediatric patients with a do-not-intubate and comfort-measures-only order | 87 | |
| HFNC as a starting therapy for mild-moderate bronchiolitis in the hospital ward | 50 | |
| HFNC to avoid therapeutic intensification in mild-moderate bronchiolitis in the pediatric ward | 50 | |
| HFNC in patients with bronchiolitis in pediatric intensive care | 72 | |
| HFNC in patients with bronchospasm in pediatric intensive care | 72 |
ARF: acute respiratory failure; FIO2: fraction of inspired oxygen; HFNC: high-flow nasal cannula therapy; IMV: invasive mechanical ventilation; NIMV: non-invasive mechanical ventilation; NIRS: non-invasive respiratory support; PaO2: partial pressure of oxygen.
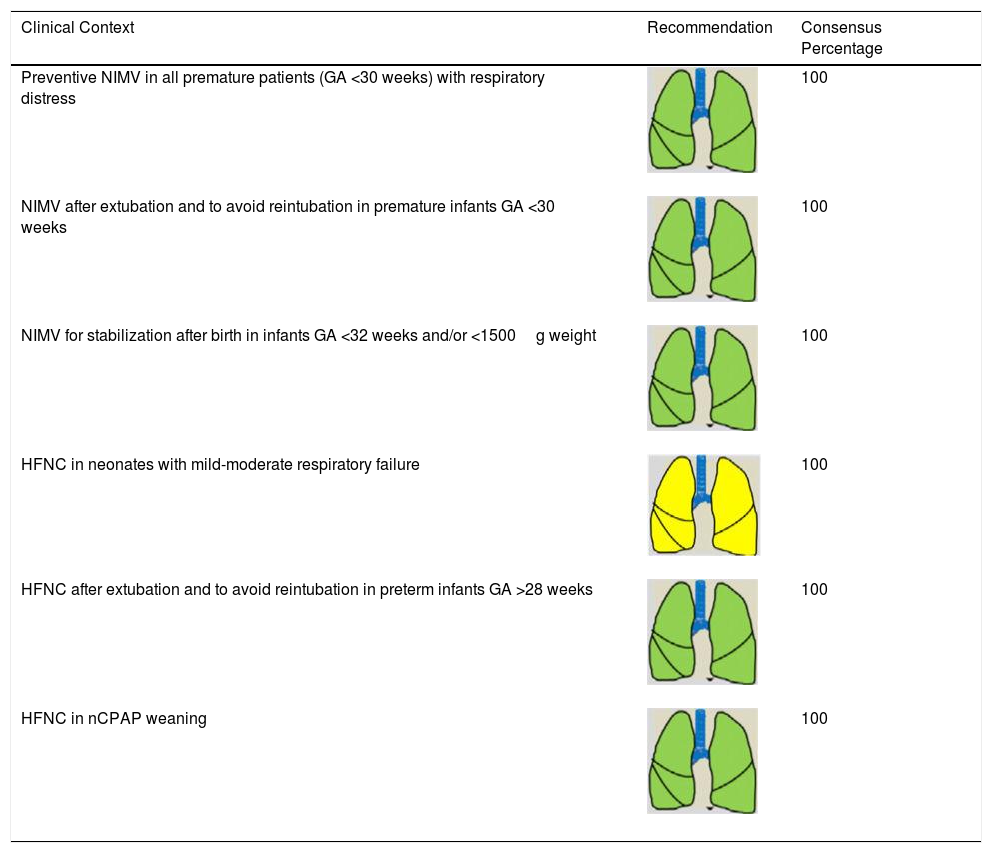
Consensus Recommendations for Indicating NIRS in Neonatal Patients.
| Clinical Context | Recommendation | Consensus Percentage |
|---|---|---|
| Preventive NIMV in all premature patients (GA <30 weeks) with respiratory distress | 100 | |
| NIMV after extubation and to avoid reintubation in premature infants GA <30 weeks | 100 | |
| NIMV for stabilization after birth in infants GA <32 weeks and/or <1500g weight | 100 | |
| HFNC in neonates with mild-moderate respiratory failure | 100 | |
| HFNC after extubation and to avoid reintubation in preterm infants GA >28 weeks | 100 | |
| HFNC in nCPAP weaning | 100 |
GA: gestational age; HFNC: high-flow nasal cannula therapy; nCPAP: nasal continuous positive nasal pressure; NIMV: non-invasive mechanical ventilation; NIRS: non-invasive respiratory support.
Consensus Recommendations for NIRS Procedure and Follow-up in Adult Patients.
| Procedure | Recommendation | Consensus Percentage |
|---|---|---|
| Stratification of NIRS candidates. Priority admission to intensive care areas for high-risk patients | 93 | |
| Use of NIMV-specific ventilators in acute patients or critical patient ventilators with NIMV module | 80 | |
| Interfaces of choice in the acute adult patient are oronasal and full-face masks | 87 | |
| Pressure ventilation in spontaneous/assisted mode is the mode of choice in acute respiratory failure | 93 | |
| Continuous monitoring of physiological parameters during the procedure (RR, HR, SpO2) | 93 | |
| Follow-up with ventilator-generated flow and pressure curve analysis | 67 | |
| Use of active humidification during NIMV | 53 | |
| NIMV efficacy assessment 1h after starting; assessment at 4–6h is a good indicator of the success/failure of the technique | 93 | |
| If there is no response to NIMV, consider early discontinuation of the technique and evaluate endotracheal intubation and invasive ventilation | 100 | |
| Immediate withdrawal of NIMV in patients with COPD exacerbation after normalization of pH and improvement of general clinical status | 53 | |
| Consider continuing NIMV in acute OHS after resolution of the acute episode, as many patients have an underlying sleep disorder | 73 | |
| Consider chronic home ventilation after an exacerbation that required NIMV in patients with restrictive lung disease, neuromuscular disease, and COPD with persistent PaCO2>56mmHg at discharge | 80 | |
| When applying HFNC, use flows between 40–50bpm in patients with moderate hypoxemic ARF with minimal FiO2 to maintain an SpO2 of about 93%–94% with humidification and temperature mentioned above | 100 |
ARF: acute respiratory failure; bpm: breaths per minutes: COPD: chronic obstructive pulmonary disease; FiO2: fraction of inspired oxygen; HFNC: high-flow nasal cannula therapy; HR: heart rate; NIMV: non-invasive mechanical ventilation; NISR: non-invasive respiratory support; OHS: obesity hypoventilation syndrome; PaCO2: partial pressure of carbon dioxide; RR: respiratory rate; SpO2: oxygen saturation.
Consensus Recommendations for NIRS Procedure and Follow-up in Pediatric Patients.
| Procedure | Recommendation | Consensus Percentage |
|---|---|---|
| In pediatric patients, clinical criteria for improvement should be evaluated 1–2h after starting the technique: decreased HR, RR, which should be in line with the patient's age, improved work of breathing (dyspnea), and decreased accessory respiratory muscle activity | 97 | |
| In pediatric patients, PaO2/FiO2 or Sao2/FiO2 should be monitored | 97 | |
| Pressure ventilation in spontaneous/assisted mode is the initial mode of choice in ARF (hypoxemia + hypercapnia) | 74 | |
| CPAP mode is the initial mode of choice in hypoxemic type I ARF without hypercapnia, and no respiratory distress | 74 | |
| Use of active humidification in the pediatric patient | 94 | |
| It is essential to maintain correct patient positioning and nasogastric tube placement during NIRS | 64 | |
| In acute-phase NIMV in the pediatric patient, the PS level should be about 4–5 cmH2O above the expiratory pressure level (PEEP or EPAP). Once patient tolerance has been achieved, the PS level can be increased in increments of 2 cmH2O until work of breathing is reduced | 94 | |
| Withdrawal of NIMV in children depends on the disease for which it was indicated, and can be undertaken more quickly when the time of progression is shorter | 69 | |
| Chronic non-invasive home support may be required in pediatric neuromuscular patients | 97 |
ARF: acute respiratory failure; CPAP: continuous positive pressure; EPAP: expiratory positive airway pressure; FiO2: fraction of inspired oxygen; HR: heart rate; NIMV: non-invasive mechanical ventilation; NIRS: non-invasive respiratory support; PaO2: partial pressure of oxygen; PEEP: positive end-expiratory pressure; PS: pressure support; RR: respiration rate; SaO2: oxygen saturation.
Consensus Recommendations for NIRS Procedure and Follow-up in Neonatal Patients.
| Clinical Context | Recommendation | Consensus Percentage |
|---|---|---|
| Use of active humidification in the neonatal patient | 100 | |
| The interface of choice in neonatology is short binasal cannulas or a nasal mask | 100 | |
| For ventilators of choice in neonatal NIMV, variable flow generators are preferable to continuous flow generators | 100 | |
| FIO2 according to SatO2 for GA | 100 | |
| Maintain good patient positioning and orogastric tube placement | 100 | |
| Criteria for failure include need for an FiO2 of 0.4–0.5 to achieve target saturation based on GA, progressive dyspnea, apneas, and persistent respiratory acidosis | 100 | |
| Start NIMV withdrawal if FiO2 <0.4, no apneas and/or bradycardias, and no clinical signs of respiratory distress | 100 |
FiO2: fraction of inspired oxygen; GA: gestational age; NIMV: non-invasive mechanical ventilation; PaO2: partial pressure of oxygen; SatO2: arterial oxygen saturation.
- •
For a patient with dyspnea and signs of severe respiratory failure of unknown cause, the use of NIMV may provide the necessary time to gather essential information about the patient's causal diagnosis, prognosis, and baseline status, and to adapt the therapeutic effort. Thus, in patients with moderate-severe dyspnea, tachypnea, and signs of labored breathing, NIMV can be initiated in the absence of contraindications or an indication for immediate urgent intubation. The evaluation of the diagnosis and prognosis of the disease causing ARF should then continue without delay. In terms of blood gases (if a determination is available), a need for FiO2 greater than 0.4 to achieve adequate oxygenation or the presence of acute ventilatory failure (pH 7.35 with PaCO2 >45mmHg) suggest that the patient is a candidate for NIMV.2,3
- •
The use of NIMV with pressure support (PS) in COPD patients with pH <7.35 and PaCO2 >45mmHg reduces the risk of intubation, hospital stay, and mortality compared to standard medical treatment.4–7 Its benefit in exacerbations that present without respiratory acidosis has not been established.8,9 It is also suggested that it can be used safely in the prehospital setting following the recommendations in the preceding section.10
- •
The use of positive pressure support (CPAP or NIMV with PS) reduces the rate of intubation in patients with acute cardiogenic pulmonary edema (ACPE) and is also associated with a reduction in mortality.11 When CPAP and NIMV with PS are compared, no data are available to support the superiority of one over the other in terms of patient progress, although patients treated with NIMV appear to show a more rapid improvement in some clinical variables.12–14 The use of CPAP in ACPE in the prehospital setting is also effective, and the need for intubation is reduced.15
- •
NIMV is recommended in patients with decompensated obesity hypoventilation syndrome (OHS) and respiratory acidosis.16
- •
A short NIMV trial in patients with severe asthma exacerbations may be performed, provided each case is evaluated individually and response is strictly monitored (ideally in an intensive care unit setting).17
- •
An NIMV trial is recommended in patients with ARF due to pneumonia and cardiorespiratory comorbidity, but it is not advisable in patients without this comorbidity.18
- •
Early NIMV may be an alternative to consider in patients with immunosuppression and ARF, although few good quality comparative studies with HFNC are available.
- •
The use of NIMV cannot be recommended in hypoxemic ARF caused by acute respiratory distress syndrome (ARDS),19 except in mild cases and in an intensive care unit setting.20
- •
The use of NIMV is suggested in patients with a do-not-intubate order if they belong to one of the diagnostic groups in which this mode has been seen to be effective, provided that the sensation of dyspnea improves during the procedure.21 The possibility exists of administering NIMV as an adjuvant treatment for dyspnea in palliative patients.22,23
- •
In patients with chronic respiratory failure due to neuromuscular and chest wall disorders, NIMV support is recommended to prevent and treat respiratory acidosis in case of exacerbation for any cause (especially infections).24
- •
The use of NIMV is suggested in patients with ARF and chest trauma in the absence of undrained pneumothorax.9
- •
The use of NIMV can be considered during viral pandemics in carefully selected patients treated in experienced centers in a protected environment with negative pressure rooms.25,26 Some indications and specific procedures for SARS-CoV-2 infection have also been proposed.27
- •
In the context of weaning from invasive mechanical ventilation (IMV) in patients with risk factors for extubation failure (especially COPD patients), a strategy for the application of post-extubation NIMV reduces the rate of reintubation, especially when combined with HFNC. However, in populations without such risk factors, NIMV cannot be recommended as a general strategy for mechanical ventilation weaning. Post-extubation ARF, however, should be treated with NIMV.28
- •
NIMV appears to be useful in ARF after abdominal and cardiothoracic surgery, and no significant adverse effects associated with surgical anastomosis have been reported.29,30
- •
The use of NIMV is recommended in any pediatric patient with ARF who presents no contraindications.31,32
- •
The use of NIMV is recommended in pediatric patients in any situation that carries a high risk of IMV weaning failure (including patients with neuromuscular disease), in order to avoid reintubation.31,32
- •
An NIMV trial is recommended in patients with moderate non-hypercapnic ARF with no associated organ failure, and also in immunosuppressed patients with ARF.33–35
- •
NIMV is recommended in patients with moderate or severe ARF associated with viral infections, mainly viral bronchiolitis.36–38
- •
NIMV is recommended in pediatric patients with ARF in the setting of a severe asthma exacerbation.39–41
- •
Patients of pediatric age who use home NIMV and need to be transferred to the hospital for ARF should continue their NIMV during the transfer process.
- •
In generic terms, any neonate born at term or preterm with respiratory disease and increased work of breathing and mild-moderate oxygenation and/or ventilation changes would be a potential candidate for NIMV.42
- •
Important points in prematurity:
- 1.
Initial stabilization after birth (gestational age [GA] <32 weeks and/or <1500g weight).
- 2.
Respiratory distress syndrome (typical in premature babies due to surfactant deficiency).
- 3.
Prevention of ARF after extubation (GA <30 weeks).43
- 1.
- •
HFNC is recommended as the first NIRS technique in patients with severe pneumonia and/or ARDS, before standard oxygen therapy and NIMV in patients with no immediate indication for orotracheal intubation.19
- •
It has been suggested that HFNC can be used in patients with ARF and immunosuppression,44 although few high-quality studies comparing HFNC with NIMV are available.
- •
The use of HFNC after scheduled extubation can be considered in patients without hypercapnia and at low risk of reintubation.45
- •
The routine use of HFNC to prevent reintubation in patients without hypercapnia and at high risk of reintubation cannot be recommended, except when combined with NIMV.45,46
- •
The use of HFNC as a therapeutic alternative to NIMV can be considered after cardiothoracic surgery in patients with postoperative respiratory failure or a high risk of reintubation.47
- •
Preoxygenation techniques with NIMV and/or HFNC instead of standard oxygen therapy in hypoxemic patients who are scheduled for intubation are suggested in order to reduce the risk of peri-intubation hypoxemia. Combining NIMV and HFNC techniques should be reserved for severely hypoxemic patients with a high risk of early desaturation during the intubation procedure (morbidly obese patients).48,49
- •
The use of HFNC as a starting or rescue therapy for mild-moderate bronchiolitis50,51 in the hospital ward to avoid admission to the pediatric intensive care unit (PICU) cannot be recommended.
- •
The use of HFNC is not recommended in patients with bronchospasm.
- •
The use of HFNC instead of CPAP in the PICU is not recommended.
- •
HFNC is used in respiratory diseases with mild-moderate respiratory failure. There is evidence and consensus that HFNC can be used during weaning from nasal CPAP and ventilatory weaning to prevent reintubation in the preterm patient of GA >28 weeks.52
- •
In general, candidates for NIRS should be stratified according to their risk of failure, on the basis of the disease that led to its indication and their clinical status. Patients with a high risk of failure, who therefore warrant extra dedication and resources, should be evaluated from the outset by a specialist working in a setting that can provide adequate monitoring and immediate provision of advanced life support measures.
- •
Units that administer NIRS should have a physician:patient ratio of no more than 1:6 and a nurse:patient ratio of no more than 1:4. Minimal continuous monitoring (pulse oximetry, ECG) 24h a day is also essential, regardless of the hospitalization area.
- •
The ventilators used should be designed specifically for NIMV and should be user-friendly, as they are often used in units with a high staff rotation rate. Ventilators for critical patients can also be used, provided they have specific algorithms for NIMV. The use of home ventilators is not recommended in acute patients, except in patients using home ventilation who progressed well on their ventilator during the acute phase. In pediatric patients, it is advisable where possible to use equipment that has been approved for use in patients up to 5kg in weight. In neonatology, variable flow generators are preferable to continuous flow generators.
- •
Selecting the interface is essential for the correct application of the technique, as this is the element that most frequently affects patient well-being and leads in a high percentage of cases to NIMV rejection. In adults, in general, the interfaces of choice are oronasal masks or full-face masks. It is advisable to have several alternatives on hand, especially for patients who due to their characteristics fail to adapt adequately to the one initially chosen as theoretically ideal. During the recent SARS-CoV-2 pandemic, positive experiences have been reported with helmet interfaces, even with different patient positions such as the combination of NIMV and HFNC in prone positioning.53,54 In neonatology, the interfaces of choice are short binasal cannulas.
- •
Active humidification systems that do not increase resistance or dead space in the system are recommended in NIMV. This recommendation is especially important in very young pediatric patients and in newborns, in whom high flows can dry the airway and make it difficult to control body temperature.
- •
In acute phase NIMV, pressure-controlled ventilation (PS) is mainly used because it can compensate for leaks and patient synchronization is straightforward.55 The objective of ventilator programming is to achieve a tidal volume greater than 300ml (or 5ml/kg ideal weight) and a spontaneous respiration rate (RR) of less than 25bpm in the adult patient. In the pediatric patient, the PS level should be about 4–5 cmH2O above the expiratory pressure level (PEEP or EPAP). Once patient tolerance has been achieved, the PS level can be increased in increments of 2 cmH2O until the work of breathing is reduced. In the neonatal patient, inspiratory pressure is usually adjusted to about 2–4 points above the PEEP level, which in turn is usually 5–6cmH2O, and may vary depending on the alveolar recruitment required and hemodynamic tolerance.
- •
The goal when programming HFNC in adults is considered to be a gas flow of around 45–50l/min. For oxygenation, a minimum FiO2 should be programmed to maintain SpO2 at about 93%–94% – or 88%–89% in cases of concomitant chronic lung disease – and a conditioning temperature of around 37°C. The use of the ROX index (SpO2/FiO2:RR) is useful for monitoring effectiveness. A value of ≥4.88 is associated with a higher probability of success.56,57
- •
In pediatrics, as a general rule in infants under 1 year of age, HFNC should be programmed at ≥2l/min and adjusted for body weight, using the formula of 2l/kg/min up to 8–10kg weight. In older children, flows should always be greater than 6l/min, and rates of up to 20 or 30l/min, approaching the equivalent of 1l/kg/min, can even be used.58 FiO2 must be set for a peripheral oxygen saturation target of 92%–97%. Temperature, by default, is around 37°C for optimal humidification.
- •
In neonates, HFNC is used with an initial flow rate of 4–8l/min. When clinical and blood gas objectives are achieved, reducing the rate to 2l/min can begin, at which stage the cannulas can be withdrawn.
- •
In adult patients, NIMV effectiveness is determined by monitoring a number of physiological parameters (level of consciousness, RR, heart rate [HR], SaO2) and blood gases within an hour of starting therapy. Tidal volume monitoring in pressure-controlled mode is also important for detecting and treating both hypoventilation and high tidal volumes, which can worsen ventilator-induced lung injury.59 Advanced monitoring using flow and pressure time curves generated by the ventilator can also be useful, if available.
- •
There are 3 types of NIMV failure: immediate failure (within the first hour of starting the procedure, attributable to poor tolerance); early failure (between 1 and 48h, the most frequent of the 3); and late failure (after 48h, mostly attributable to nosocomial infection).
- •
In addition to determining blood gases on initiation of the technique, the 4–6h efficacy assessment is a good indicator of NIMV success/failure. If there is no improvement in the clinical picture (improved encephalopathy, reduced work of breathing, reduced respiratory rate, reduced dyspnea scale score) or blood gases (persistent or increased respiratory acidosis or hypoxemia, depending on the type of respiratory failure) within this time period, the technique must be considered to have failed.
- •
NIMV failure requires a change in strategy, and orotracheal intubation must be immediately considered, especially in patients with hypoxemic ARF. In certain situations, changes in ventilator programming, interface, medical treatment, etc. may be considered, while remembering that any maneuver that unnecessarily prolongs the duration of NIMV failure can lead to increased mortality.
- •
In patients with COPD and respiratory acidosis, NIMV can be withdrawn as soon as the acute episode has resolved, pH has normalized, both PaCO2 and the patient's overall status have improved, and spontaneous breathing is tolerated without ventilatory support.60 If hypercapnia (>56mmHg) persists 2–4 weeks after hospital discharge, chronic home ventilation should be considered.61
- •
In patients with neuromuscular or chest wall disorders, NIMV may require a more progressive withdrawal, as complete withdrawal may be difficult in many cases, and a transition to chronic home NIMV may be necessary.
- •
Patients with obesity hypoventilation syndrome should continue nocturnal NIMV for a few more days, and the indication of CPAP or long-term home nocturnal ventilation may be considered.
- •
In pediatric patients, the objectives do not differ from the adult patient, and clinical criteria for improvement 1–2h after initiation of the technique include: decreased HR and RR, which should be in line with the patient's age and lower than at the start of the technique, improved work of breathing (dyspnea), and decreased accessory respiratory muscle activity. A number of respiratory distress scales (Pulmonary Score, Wood-Dowes, Tussing, etc.) are also used to assess progress.
- •
NIMV withdrawal in children depends on the disease for which it was indicated. Asthma patients, for example, generally need support for 48–72h, bronchiolitis patients between 5 and 7 days, and neuromuscular patients may subsequently need non-invasive chronic support at home.
- •
In neonatology, patient positioning with appropriate airway alignment is particularly important if the technique is to succeed. The need for an FiO2 of 0.4–0.5 to achieve target saturation based on GA, progressive dyspnea and apneas, and persistent respiratory acidosis are considered indicative of failure.
- •
To withdraw NIMV in the neonatal patient, oxygen requirements to maintain adequate oxygenation should be below 0.4, and the patient should not present apnea, bradycardias, or labored breathing.62
We have developed clinical practice recommendations for the current use of NIMV/HFNC for ARF in adult and pediatric-neonatal patients. ARF in the adult and pediatric-neonatal patient should be managed taking into account the heterogeneity of the clinical scenarios with respect to indications, stratification, and follow-up in the use of NIMV and HFNC. The recommendations contained in this document reflect for the first time the degree of agreement between the main scientific societies. This consensus document provides an up-to-date working tool for all physicians responsible for the management of adult and pediatric-neonatal patients with ARF and will help reduce clinical variability in the care of these patients.
It is highly likely that some of these recommendations will be modified over time as the scientific knowledge in this field grows and becomes established, especially with regard to the role of NIMV and HFNC in relation to new emerging technologies such as oxygen and extracorporeal extraction of CO2. A systematic, dynamic, and integrative approach is needed to improve the management of this prevalent problem, in order to reduce the significant burden of hospital care and improve patient outcomes.
Please cite this article as: Luján M, Peñuelas Ó, Cinesi Gómez C, García-Salido A, Moreno Hernando J, Romero Berrocal A, et al. Sumario de recomendaciones y puntos clave del Consenso de las Sociedades Científicas Españolas (SEPAR, SEMICYUC, SEMES; SECIP, SENeo, SEDAR, SENP) para la utilización de la ventilación no invasiva y terapia de alto flujo con cánulas nasales en el paciente adulto, pediátrico y neonatal con insuficiencia respiratoria aguda grave. Arch Bronconeumol. 2021;57:415–427.
In agreement with the authors and editors, this article has been published in Medicina Intensiva (https://doi.org/10.1016/j.medin.2020.08.016).