Studies on inflammation biomarkers in serum and in exhaled breath condensate (EBC) in obstructive sleep apnea (OSA) have shown conflicting results. The objective of this study is to assess EBC and serum biomarkers in OSA patients at baseline and after continuous positive airway pressure (CPAP) or upper airway surgery (UAS).
Patients and methodsNine OSA patients referred for UAS were matched for anthropometric characteristics and apnea–hypopnea index with 20 patients receiving CPAP. pH, nitrite (NO2−), nitrate, and interleukin 6 in EBC and NO2−, nitrate, leukotriene B4, and interleukin 6 in serum were determined. EBC and serum samples were collected at baseline and 3 months after CPAP or UAS.
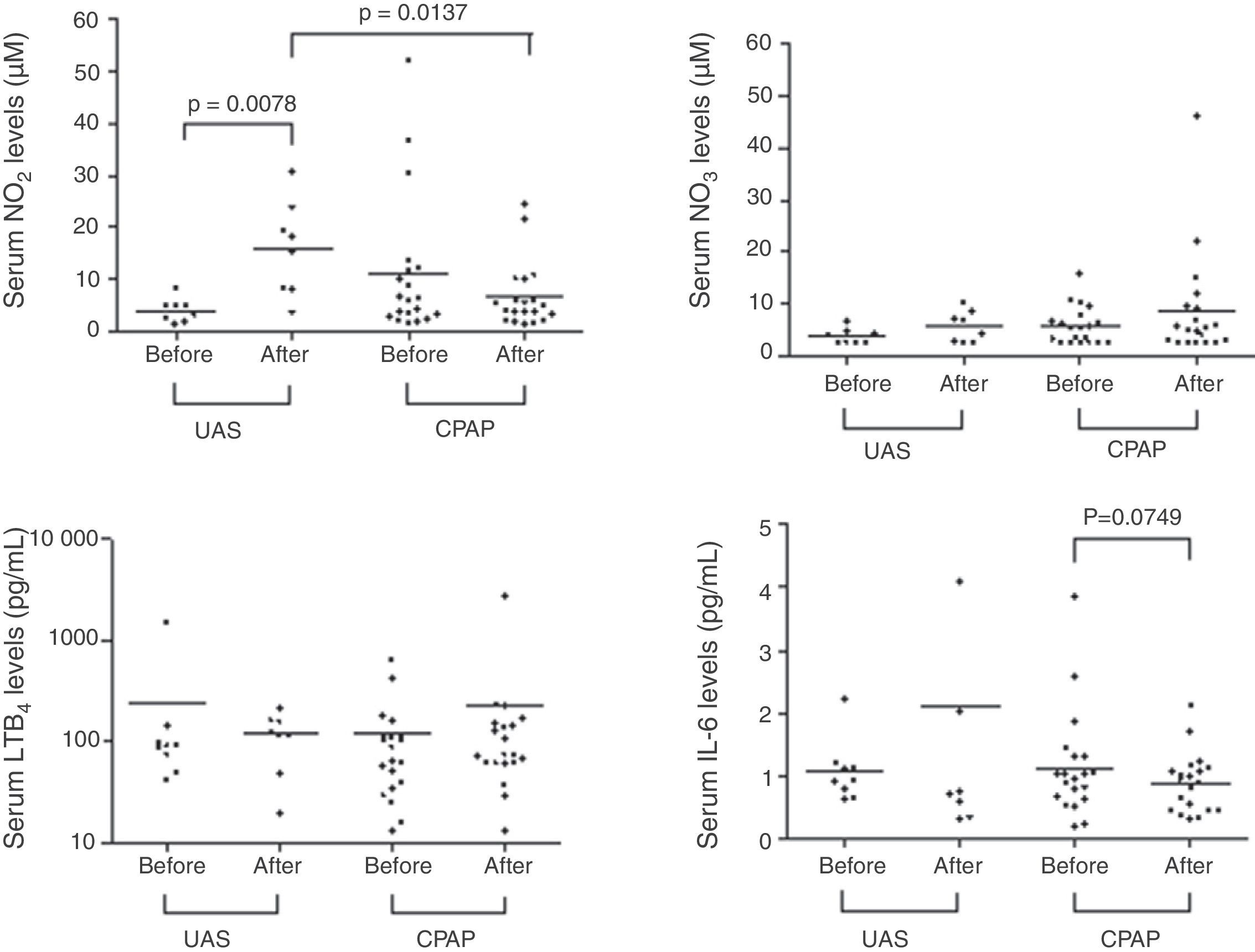
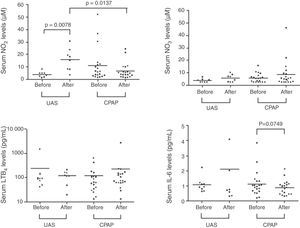
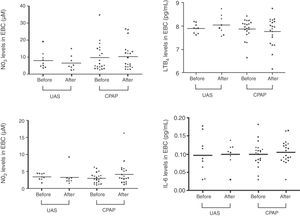
ResultsPatients’ mean body mass index was 30 (range 24.9–40) kg/m2. EBC biomarker levels at baseline were within normal range and did not differ significantly after CPAP or UAS. No significant changes were observed in the serum concentration of the biomarkers determined after CPAP but the serum concentration of NO2− increased significantly at 3 months after UAS (P=.0078).
ConclusionIn mildly obese OSA patients, EBC biomarkers of inflammation or oxidative stress were normal at baseline and remained unchanged 3 months after UAS or CPAP. Although UAS was not effective in terms of reducing OSA severity, it was associated with an increase in serum NO2−.
Los estudios de los biomarcadores inflamatorios en suero y en el condensado de aire exhalado (CAE) en la apnea obstructiva del sueño (AOS) han producido resultados contradictorios. El objetivo de este estudio es evaluar los biomarcadores en CAE y en suero en pacientes con AOS en la situación basal y después de la aplicación de presión positiva continua de vías aéreas (CPAP) o de cirugía de las vías aéreas superiores (CVAS).
Pacientes y métodosNueve pacientes con AOS que fueron remitidos para CVAS fueron emparejados según sus características antropométricas y el índice de apnea-hipopnea con 20 pacientes que fueron tratados con CPAP. Se efectuaron determinaciones de pH, nitrito (NO2−), nitrato e interleucina 6 en CAE, y de NO2−, nitrato, leucotrieno B4 e interleucina 6 en suero. Se obtuvieron muestras de CAE y de suero en la situación basal y 3 meses después de la CPAP o la CVAS.
ResultadosEl valor medio del índice de masa corporal de los pacientes fue de 30 (rango 24,9–40) kg/m2. Los niveles de marcadores en CAE en la situación basal estuvieron dentro del rango normal y no presentaron diferencias significativas tras la CPAP o la CVAS. No se observaron cambios significativos en las concentraciones séricas de los biomarcadores evaluados tras la CPAP, pero la concentración sérica de NO2− aumentó significativamente a los 3 meses de la CVAS (p=0,0078).
ConclusiónEn los pacientes con AOS y obesidad leve, los biomarcadores de la inflamación o el estrés oxidativo en el CAE presentaron unos niveles basales normales y se mantuvieron inalterados 3 meses después de la CVAS o la CPAP. La CVAS, aunque no resultó efectiva por lo que respecta a la reducción de la gravedad de la AOS, se asoció a un aumento de los niveles séricos de NO2−.
There is a growing body of evidence linking inflammatory processes, oxidative stress and endothelial dysfunction with obstructive sleep apnea (OSA) and morbidity and mortality rates.1 Exhaled breath condensate (EBC) is a noninvasive means of studying inflammatory biomarkers, mainly in asthma patients.2,3 Some studies have focused on the usefulness of EBC in evaluating the presence of inflammation and oxidative stress in OSA patients.4–10 These factors are important since the mechanical trauma caused by snoring and repeated upper airway collapse leads to local inflammation that can spread to the respiratory system and the systemic circulation, thereby contributing to the pathogenesis and continuance of OSA. Patients with severe OAS present increased proinflammatory cytokine expression in the mucosal or muscle compartments of the upper airways, accompanied by increased connective tissue deposition.11 However, the extent to which these anomalies contribute to upper airways dysfunction in OSA and systemic inflammation is still unclear. Previous studies have shown that continuous positive airway pressure (CPAP) reduces high levels of inflammatory and oxidative stress biomarkers in serum, although results are inconclusive.12–15
CPAP is the treatment of choice for patients with moderate to severe OSA, although some reject this therapy in favor of surgical treatment to enlarge the upper airways. Although upper airway surgery (UAS) for the treatment of OSA is controversial, we have reported increased survival rates in patients undergoing UAS to treat severe OSA compared to untreated patients,16 while another study reported lower tumor necrosis factor-alpha (TNF-α) levels after UAS, thereby possibly ameliorating the systemic inflammation and preventing the development of cardiovascular consequences.17
The aim of this study was to evaluate the effects of two treatments for OSA based on the hypothesis that both CPAP and UAS can improve local inflammation (pH, interleukin-6 [IL-6], nitrite [NO2−], nitrate [NO3−]), systemic inflammation (IL-6, leukotriene B4 [LTB4]), and endothelial function (NO2−, NO3−) in patients with OSA.
Materials and MethodsStudy PopulationNine patients with OSA (apnea hypopnea index [AHI]>5/h) selected by an otolaryngologist for UAS were matched by age, sex, body mass index (BMI), and AHI with 20 patients with OSA referred to sleep units and offered CPAP if the AHI was >5/h. Patients were included in the study from April 2008 to April 2009.
Inclusion criteria were: never-smoker or ex-smoker for more than 3 months prior to the start of the study; not taking anti-inflammatory treatment (inhaled, nasal, oral or injectable) for 4 weeks prior to the start of the study; and no endocrine disorder or any other known cause of sleep disturbance (disease, drug therapy or other interventions). Other possible causes of upper or lower airway inflammation (allergic rhinitis, asthma or chronic obstructive pulmonary disorder) were ruled out on the basis of the medical history and pulmonary function test results. Patients with obstructive lung diseases (forced expiratory volume in one second [FEV1]/forced vital capacity [FVC]≤0.7) were excluded. Patients’ height (cm) and weight (kg) were measured and BMI was calculated (kg of body weight/height [m2]).
Criteria for UAS were AHI>5 and fewer than 40 episodes/h in patients with upper airway morphological alterations (velopharyngeal insufficiency) who had refused CPAP therapy. Patients with BMI>35kg/m2 or craniofacial abnormalities such as micrognathism and prognathism were ruled out for UAS. The UAS procedure consisted in uvulopalatopharyngoplasty.
The study was approved by the hospital independent ethics committee, and written informed consent was obtained from all subjects.
Respiratory PolygraphyNasal airflow, pulse oximetry, respiratory effort, body position, and snoring were monitored overnight using the Somnea device (Compumedics, Abbotsford, Australia). Apneas were defined as the total absence of nasal airflow for at least 10s, and hypopneas as a significant reduction in nasal airflow for at least 10s associated with 3% oxygen desaturation. Obstructive apneas were distinguished from central apneas by respiratory effort channels (presence or absence of thoracic and abdominal movement). AHI was obtained by dividing the total number of apneas and hypopneas by the total recorded sleep time. OSA was defined as an AHI≥5. Patients with an AHI of between 5 and 30 were classified as mild to moderate OSA, and those with AHI>30 were considered to have severe OSA.
Continuous Positive Airway Pressure TitrationIn patients in the CPAP group, pressure was set using an automatic positive pressure titration device (AutoSet Spirit™; ResMed, Sydney, Australia). The optimal pressure setting was determined visually based on the printed data reports.18 Following this, patients were prescribed CPAP, which was provided free of charge by the Spanish National Healthcare System.
Pulmonary Function TestsFEV1, FVC, and the FEV1/FVC ratio were determined using a spirometer (MasterScreen™ PFT; Jaeger, Höchberg, Germany).
Serum SamplesSerum samples were collected by centrifuging whole blood. Each serum sample was divided into 500μL aliquots. Aliquots used for determining NO2−, NO3−, LTB4, and IL-6 levels were stored immediately at −70°C, and analyzed within 1 month of collection.
Exhaled Breathing Condensate CollectionEBC was obtained during normal breathing using a commercial condenser (EcoScreen™; Jaeger, Wurzburgo, Germany), as described previously.19 Briefly, exhaled air enters and leaves the chamber through one-way valves while the chamber is kept closed. Patients breathe tidally into the condenser through a mouthpiece while wearing a nose clip. Throughout the collection process the condenser chamber is maintained at a low temperature to cool the sample. Blood samples were taken using test tubes that have been disinfected for 30min in sodium dichloroisocyanurate (Inibsa Lab, Barcelona, Spain), and washed for 24h in distilled water and for 2h with ultrapure water (Fresenius Kabi, Barcelona, Spain). Breathing patterns were determined by spirometry (EcoVent; Jaeger, Wurzburgo, Germany) connected to the expiratory valve of the device. The spirometer recorded timed total expiration, tidal volume, minute ventilation and breathing rate. Patients were asked to fast for 2h prior to sample collection. A total volume of 150L of exhaled breath was collected from each patient. Each EBC sample was divided into 500μL aliquots and stored in 2–5 plastic tubes. The aliquots used for NO2−, NO3−, and IL-6 determination were stored immediately at −70°C and analyzed within 1 month of collection. One aliquot was used for pH determination immediately following collection.
Determination of pH in Exhaled Breath CondensateThe pH level of the EBC in one of the aliquots was determined to within ±0.01 pH using a calibrated pH meter (GLP 21; Crison Instruments, S. A., Barcelona, Spain) and an electrode for small samples (Crison 50 28; Crison Instruments, S. A., Barcelona, Spain) after helium deaeration (350mL/min for 10min). The electrode was calibrated daily using pH 7.02 and 4.00 reference buffers.20
Determination of Nitrite and NitrateNO2− and NO3− levels in serum and EBC samples were determined by Griess colorimetric assay. Total NO2−/NO3− (converted NO3− plus NO2−) was determined in duplicate samples using Griess reagent (Cayman Chemical Company, Ann Arbor, MI, USA). Levels were determined at an absorbance of 540nm using a microplate reader. Assay sensitivity was 1μM for NO2− and 2.5μM for NO3−. Intra- and inter-assay coefficients of variation of 6% and 4%, and 9% and 5%, for NO3− and NO2− respectively, were noted. We decided to determine the end products of NO metabolism instead of exhaled NO in order to compare compounds generated from the same sample.
Determination of Interleukin 6 and Leukotriene B4IL-6 levels in serum and EBC were determined by means of a commercial high sensitivity enzyme-linked immunosorbent assay kit (Bender MedSystems GmbH, Vienna, Austria), with a sensitivity of 0.03pg/mL. The intra- and inter-assay coefficients of variation were 4.9% and 6.0%, respectively.
Serum LTB4 levels were determined using a commercial LTB4 enzyme-linked immunosorbent assay kit (Cayman Chemical Company, Ann Arbor, IM, USA), with a sensitivity of 13pg/mL.
ProtocolOvernight respiratory polygraphy was performed on all patients at baseline and then at 3 months in patients undergoing UAS. EBC and serum samples were collected at baseline and 3 months after UAS or after the start of CPAP.
Sample SizeAssuming an alpha risk of 0.05 and a beta risk of <0.2 in a two-tailed test, seven patients would be needed in each group in order to detect a difference of ≥0.58 in pH in EBC. Based on the results obtained by Petrosyan et al.,8 a standard deviation of 0.44 was assumed.
Data AnalysisThe primary endpoint of the study was changes in EBC and serum biomarkers using CPAP and UAS at 3-months follow-up. As a secondary endpoint we analyzed the correlation between EBC and serum biomarkers and severity of OSA based on AHI. The one-sample Kolmogorov–Smirnov test was used to evaluate goodness of fit. The Mann–Whitney and Wilcoxon signed-rank tests were used to analyze inter- and intra-group differences, respectively. Spearman's rank correlation coefficient was used to determine correlations between AHI and study biomarkers. Undetectable levels of NO2− and NO3− were assigned the limit of detection values (1μM and 2.5μM, respectively). Undetectable levels of IL-6 or LTB4 were given limit of detection values (0.03pg/mL and 13pg/mL, respectively). Data were expressed as absolute and median values, where appropriate. All statistical analysis was performed using SPSS® version 17.0 for Windows® (SPSS, Inc., Chicago, IL, USA).
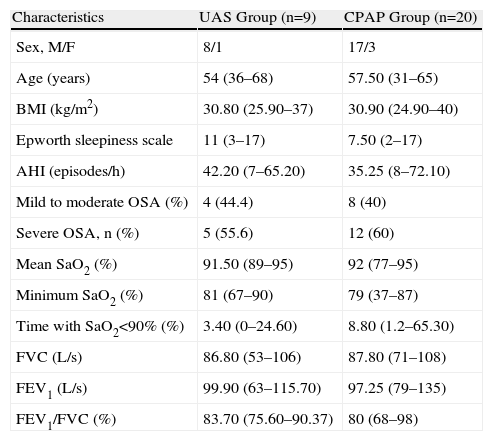
ResultsThe demographic data and clinical characteristics of the patients are summarized in Table 1. There were no significant differences between the CPAP and UAS groups in terms of anthropometric characteristics, sleep respiratory parameters or pulmonary function.
Baseline Characteristics and Sleep and Pulmonary Function Data of Study Subjects.
| Characteristics | UAS Group (n=9) | CPAP Group (n=20) |
| Sex, M/F | 8/1 | 17/3 |
| Age (years) | 54 (36–68) | 57.50 (31–65) |
| BMI (kg/m2) | 30.80 (25.90–37) | 30.90 (24.90–40) |
| Epworth sleepiness scale | 11 (3–17) | 7.50 (2–17) |
| AHI (episodes/h) | 42.20 (7–65.20) | 35.25 (8–72.10) |
| Mild to moderate OSA (%) | 4 (44.4) | 8 (40) |
| Severe OSA, n (%) | 5 (55.6) | 12 (60) |
| Mean SaO2 (%) | 91.50 (89–95) | 92 (77–95) |
| Minimum SaO2 (%) | 81 (67–90) | 79 (37–87) |
| Time with SaO2<90% (%) | 3.40 (0–24.60) | 8.80 (1.2–65.30) |
| FVC (L/s) | 86.80 (53–106) | 87.80 (71–108) |
| FEV1 (L/s) | 99.90 (63–115.70) | 97.25 (79–135) |
| FEV1/FVC (%) | 83.70 (75.60–90.37) | 80 (68–98) |
CPAP: continuous positive airway pressure; UAS: upper airway surgery; F: female; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; AHI: apnea hypopnea index; BMI: body mass index; M: male; SaO2: arterial oxygen saturation.
Data are presented as n or median (range), unless otherwise indicated.
No significant differences were found between baseline respiratory polygraphy and the same test performed 3 months after UAS. In patients receiving CPAP, median (range) use was 4.9h (0.33–6.7) with a median (range) CPAP setting of 9cmH2O (7–13).
No significant correlation was found between EBC and serum biomarker levels and severity of OSA.
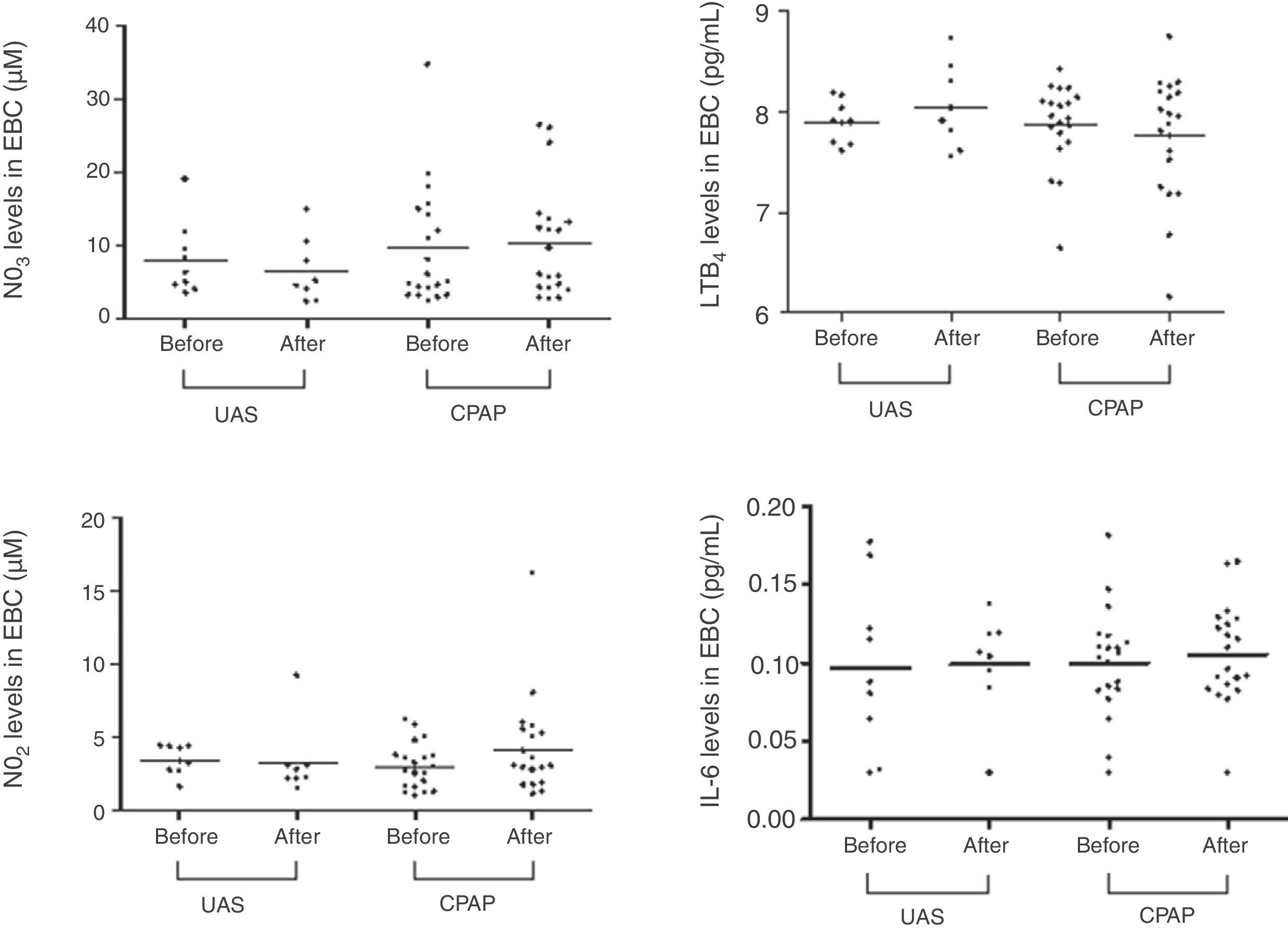
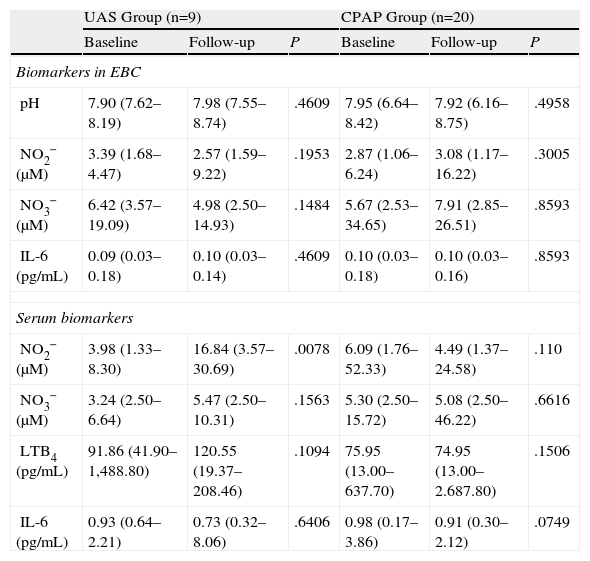
Table 2 and Figs. 1 and 2 show EBC and serum biomarker levels at baseline and after 3-months follow-up. No significant differences were found between EBC biomarker levels at baseline and 3-months follow-up, or between patients receiving CPAP and undergoing UAS. Serum samples showed significantly increased NO2− levels in the UAS group 3-months post-surgery (P=.0078), with serum NO2− levels being higher in the UAS group vs the CPAP group (P=.0137). Serum IL-6 levels tended to fall after 3 months CPAP therapy (P=.0749). No significant differences were found between other serum biomarkers.
Determinations in Serum and Exhaled Breath Condensate.
| UAS Group (n=9) | CPAP Group (n=20) | |||||
| Baseline | Follow-up | P | Baseline | Follow-up | P | |
| Biomarkers in EBC | ||||||
| pH | 7.90 (7.62–8.19) | 7.98 (7.55–8.74) | .4609 | 7.95 (6.64–8.42) | 7.92 (6.16–8.75) | .4958 |
| NO2− (μM) | 3.39 (1.68–4.47) | 2.57 (1.59–9.22) | .1953 | 2.87 (1.06–6.24) | 3.08 (1.17–16.22) | .3005 |
| NO3− (μM) | 6.42 (3.57–19.09) | 4.98 (2.50–14.93) | .1484 | 5.67 (2.53–34.65) | 7.91 (2.85–26.51) | .8593 |
| IL-6 (pg/mL) | 0.09 (0.03–0.18) | 0.10 (0.03–0.14) | .4609 | 0.10 (0.03–0.18) | 0.10 (0.03–0.16) | .8593 |
| Serum biomarkers | ||||||
| NO2− (μM) | 3.98 (1.33–8.30) | 16.84 (3.57–30.69) | .0078 | 6.09 (1.76–52.33) | 4.49 (1.37–24.58) | .110 |
| NO3− (μM) | 3.24 (2.50–6.64) | 5.47 (2.50–10.31) | .1563 | 5.30 (2.50–15.72) | 5.08 (2.50–46.22) | .6616 |
| LTB4 (pg/mL) | 91.86 (41.90–1,488.80) | 120.55 (19.37–208.46) | .1094 | 75.95 (13.00–637.70) | 74.95 (13.00–2.687.80) | .1506 |
| IL-6 (pg/mL) | 0.93 (0.64–2.21) | 0.73 (0.32–8.06) | .6406 | 0.98 (0.17–3.86) | 0.91 (0.30–2.12) | .0749 |
EBC: exhaled breath condensate; CPAP: continuous positive airway pressure; UAS: upper airway surgery.
Data are presented as median (range), unless otherwise indicated.
In the UAS group, we observed a significant negative correlation between baseline BMI and serum NO2− levels (r=−0.814, P=.014).
DiscussionOur study determined baseline levels and the effect of UAS and CPAP on inflammatory (IL-6, NO2−, NO3−) and oxidative stress (pH) biomarkers in EBC and on inflammatory and endothelial function biomarkers (IL-6, LTB4 and NO2−, NO3−, respectively) in serum in patients with OSA. No significant correlation between EBC and serum biomarkers and severity of OSA was found. Although UAS had no effect on AHI or oxygen saturation levels, the procedure was associated with increased serum NO2− levels at 3-months follow-up. CPAP had no significant effect on EBC or serum parameters at 3 months.
With respect to EBC findings, our study has shown that pH, NO3−, and NO2− levels were within the normal age-specific reference range, as described by our group in an earlier study in healthy volunteers.21 This result contrasts with the findings of three studies that reported low pH in EBC in patients with OSA.7,8,22 In two of these studies, subjects were younger,7,22 while in all three, patients were more obese and their OSA more severe, which could explain the discrepancy in the results. Obesity can be a confounding factor in EBC biomarker determination; airway inflammation has been observed in obese patients with and without OSA compared to normal weight controls,7 while another study showed high inflammatory biomarker levels in the airways of obese patients compared to non-obese OSA patients.8
Several studies have reported high levels of IL-6, a marker of neutrophilic inflammation, in the EBC of patients with OSA,4,5,8,22 but only one group8 analyzed NO2−, NO3−, and LTB levels in the EBC of apneic patients, reporting a rise in these levels indicative of the presence of local inflammation. Agustí et al.23 showed that exhaled NO in patients with OSA (albeit using a different method) was not significantly different from that of the healthy population.
We did not find any correlation between EBC biomarker levels and severity of OSA, a finding that is contrary to the correlation between some biomarkers and polysomnography parameters observed by other authors.4,8,9,22 The use of different biomarker analysis methods, differences in anthropometric characteristics, severity of OSA, and comorbidities could explain these contradictory results.
The effect of OSA on EBC has not hitherto been described. In our series, surgery was not effective in reducing AHI or improving SaO2 levels, and was not associated with any changes in biomarker levels in EBC. The most plausible explanation for this is that baseline biomarker values (pH, NO2−, and NO3−) were already within normal limits, pointing to a floor effect,21 and the ineffectiveness of the surgical procedure.
We were unable to show any effect on biomarker levels in EBC following CPAP. Again, normal baseline levels in our patients prevented us from detecting changes after this therapy. Very few studies have evaluated the effect of CPAP on biomarker levels in EBC. Petrosyan et al.8 reported increased pH levels in EBC after 1 month of CPAP therapy, but no change in NO3−, LTB4 or 8-isoprostane. Carpagnano et al.6 described a reduction in 8-isoprostane after 2 nights of CPAP treatment. Karamanli et al.9 observed that CPAP reduced TNF-α, IL-6, 8-isoprostane, and peroxynitrite levels in EBC.
After analyzing the effect of both treatment approaches on serum, the only significant change observed 3 months after surgery was an increase in plasma NO2− levels. This new and intriguing observation could be considered beneficial, as serum NO2− levels show endothelial NO production and, by association, improved endothelial function. This could either be interpreted as a random observation, considering the small sample size, or could be attributed to a real beneficial systemic effect of UAS unrelated to AHI or SaO2, since these parameters remained unaltered in the 3-month follow-up sleep study. One hypothesis suggests it could be due to improvements in snoring and therefore NO2− levels, but this seems unlikely since CPAP, which eliminates snoring, was not seen to affect serum NO2− levels. As far as we are aware, only one study has hitherto analyzed the effect of UAS on circulating inflammatory biomarkers in OSA, finding a reduction in plasma TNF-α levels 1 week after upper airway surgery in OSA patients.17 EBC, however, showed no increased serum NO2− levels after UAS. Serum and upper airway cytokines probably have a different origin. Intermittent hypoxia and sympathetic activation stimulate cytokine expression and cause endothelial dysfunction. These mechanisms and their consequences, together with local inflammation caused by snoring and intermittent airway occlusion, can affect the upper airways. To our knowledge, only one study has previously analyzed biomarkers of inflammation, oxidative stress, and endothelial dysfunction in both EBC and serum in the same group of patients.9 In that case, it was observed that CPAP therapy was associated with a reduction of all biomarkers analyzed in EBC, although only 8-isoprostane and nitrotyrosine were reduced in serum.
We did not observe any significant changes in circulating inflammatory biomarkers or in endothelial function at 3-month follow-up after CPAP, although we did note a tendency, albeit insignificant, toward reduction of serum IL-6 levels. The effect of the confounding factor of visceral obesity could explain the contradictory results obtained in studies investigating serum IL-6, a cytokine produced in visceral adipose tissue, in OSA. Previous studies have suggested that OSA patients have increased IL-6 levels.24 In one uncontrolled study in 17 patients with moderate to severe OSA, Yokoe et al. described a significant reduction in IL-6 and C-reactive protein (CRP) after 4 weeks of CPAP,14 a result that was not confirmed by Mehra et al.25 or by the first randomized controlled trial conducted by Kohler et al.,26 which reported no change in IL-6 and interferon-gamma cytokines in 100 patients with moderate to severe OSA after 4 weeks of CPAP. Karamanli et al. showed significant reduction in serum nitrotyrosine and 8-isoprostane levels after 3 months of CPAP, although sedimentation rate, CRP, IL-6, and TNF-α remained unchanged.9
Serum NO levels were used as endothelial function markers, since circulating NO is produced directly in vascular endothelium.27 Several studies have described a reduction in circulating NO levels (NO3−, NO2−) in patients with OSA compared to non-apneic patients,28 observing that CPAP can increase NO levels.29–31 Our results do not support these observations, possibly due to differences in the methodology used to determine NO derivatives or in the characteristics of the study population.
LTB4, an inflammatory mediator, is a product of the 5-lipoxygenase pathway of arachidonic acid metabolism and is released by polymorphonuclear cells. Lefebvre et al.32 reported that LTB4 levels in non-obese OSA patients increased in relation to oxygen desaturation and decreased significantly after 3 months of CPAP, and that LTB4 production correlated with carotid luminal diameter. In contrast to these results, we were unable to identify any significant changes in serum LTB4 levels in a more overweight population, albeit with a similar follow-up period.
Aside from these considerations, the study probably lacked sufficient statistical power to detect differences in serum parameters. The CPAP group had a power of 52% for IL-6, but only 3% for NO2−. However, the study had a power of 96% for the detection of changes in NO2− in the UAS group, which could support the significant increase observed in post-operative NO2− levels.
The strong points of our study lie in careful patient selection, evaluation of UAS efficacy by means of follow-up respiratory polygraphy, and objective determination of CPAP. The limitations were absence of any effect of UAS on OSA parameters and small sample size, even though this was sufficient to detect significant differences in pH in EBC. Nevertheless, the study lacked the power necessary to detect significant changes in serum parameters. Different results might have been obtained if we had limited our selection to patients with severe OSA. UAS, however, is not a common treatment strategy, and over the course of the study only 9 patients with OSA (AHI>5 and <40) were referred by the Ear, Nose, and Throat (ENT) Unit for UAS, each of whom was matched by severity of OSA with two study patients from the Sleep Unit receiving CPAP. This was not a randomized study because, as mentioned above, few patients undergo UAS in our hospital, and it would be unethical to randomly assign some patients to an accepted treatment such as CPAP and others to the more controversial UAS strategy.
Future studies should be conducted to ascertain whether a longer follow-up period would show the beneficial effects of both treatments on EBC and serum parameters, as most studies have short-term follow-up. We suggest that since age, obesity, and severity of OSA can affect inflammation and oxidative stress, more obese patients and/or those with more severe OSA would be more likely to provide a measurable response to treatment in terms of EBC and serum biomarkers.
In summary, we observed that CPAP and UAS in mildly obese patients with OSA did not change biomarkers in EBC at 3-months follow-up. Although UAS was not successful in improving polysomnography parameters, serum NO2− levels increased significantly at 3-months follow-up, while CPAP did not produce measurable changes in serum biomarkers. Further studies are needed to define the role of UAS in OSA patients in both research and clinical practice.
AuthorsDr. Patrícia Lloberes was involved in designing the study, collecting and interpreting the data and writing the manuscript.
Sara Sánchez-Vidaurre was involved in designing the study and in collecting and interpreting the data.
Dr. Alex Ferré was involved in designing the study and in collecting and interpreting the data.
Dr. María Jesús Cruz was involved in designing the study and in collecting and interpreting the data.
Dr. Juan Lorente was involved in designing the study and in interpreting the data.
Dr. Gabriel Sampol was involved in designing the study and in interpreting the data.
Dr. Ferran Morell was involved in designing the study and in interpreting the data.
Dr. Xavier Muñoz was involved in designing the study and in interpreting the data.
Conflict of InterestThe authors have no conflicts of interest.
The authors thank María Dolores Untoria for processing EBC and blood samples, Rosa Llòria for her help in writing the manuscript, and Yvette Jusseaume for editing the article and translating it into English.
Please cite this article as: Lloberes P, Sánchez-Vidaurre S, Ferré À, Cruz MJ, Lorente J, Sampol G, et al. Efecto de la presión positiva continua en las vías aéreas y de la cirugía de las vías aéreas superiores sobre los biomarcadores en condensado de aire exhalado y en suero en pacientes con apnea del sueño. Arch Bronconeumol. 2014;50:422–428.