The main risk factor for lung cancer is smoking, a habit that varies according to age and sex. The objective of this study was to explore trends in lung cancer mortality by sex and age from 1980 to 2013 in Spain.
MethodsWe used lung cancer mortality (International Classification of Diseases code 162 for the 9th edition, and codes C33 and C34 for 10th edition) and population data from the Spanish National Statistics Institute. Crude, truncated, age-adjusted mortality and age-specific mortality rates were assessed through joinpoint regression to estimate the annual percent change (APC).
ResultsAge-adjusted mortality rate significantly increased from 1980 to 1991 among men (APC=3.12%) and significantly decreased between 2001 and 2013 (APC=−1.53%), a similar pattern was observed in age-specific rates. Among women, age-adjusted mortality rate increased from 1989 (APC 1989–1997=1.82%), with the greatest increase observed from 1997 until the end of the study in 2013 (APC=4.41%).
ConclusionsDiverging trends in the prevalence of smoking could explain the increase in the rate of lung cancer-related mortality among Spanish women since the early 1990s. Public health policies should be implemented to reduce tobacco consumption in women and halt the increase in lung cancer mortality.
El principal factor de riesgo del cáncer de pulmón es el tabaco, cuyo consumo varía según la edad y el sexo. El objetivo de este trabajo es describir la tendencia de la mortalidad por cáncer de pulmón por sexo y edad desde 1980 hasta 2013 en España.
MétodosSe utilizaron los datos de mortalidad por cáncer de pulmón (código 162 para la novena revisión de la Clasificación Internacional de Enfermedades y códigos C33 y C34 para la décima revisión) y los datos de población proporcionados por el Instituto Nacional de Estadística. Se calcularon las tasas de mortalidad bruta, truncada, ajustada por edad y específicas por edad y se estimó el cambio porcentual anual (CPA) mediante un modelo de regresión joinpoint.
ResultadosLa tasa ajustada por edad aumentó significativamente de 1980 a 1991 entre hombres (CPA=3,12%) y descendió significativamente desde 2001 hasta 2013 (CPA=−1,53%), con un patrón similar observado para tasas específicas según grupos de edad. Entre mujeres, la tasa ajustada por edad aumentó desde 1989 (CPA de 1989 a 1997=1,82%), con un aumento más pronunciado desde 1997 hasta el final del estudio en 2013 (CPA=4,41%).
ConclusionesLas tendencias divergentes en la prevalencia de tabaco podrían explicar el aumento de la mortalidad por cáncer de pulmón entre las mujeres españolas desde los inicios de los años noventa. Se deberían implementar políticas de salud pública enfocadas a ayudar a la reducción del consumo de tabaco entre las mujeres fumadoras, y detener el aumento de la mortalidad por cáncer de pulmón.
Non-communicable diseases are the main cause of morbidity and mortality in developed countries.1–3 Smoking, excessive use of alcohol, a poor diet and too little physical activity are closely associated with the risk of mortality in non-communicable diseases.1,3 Smoking is also the main cause of avoidable deaths on a global basis,4 and constitutes the principal risk factor for developing lung cancer.5 In 2000, an estimated 4.83 million premature deaths worldwide were attributed to smoking, 17% of which were due to lung cancer.6 In Spain, it has been estimated that 31% of the 53,155 deaths attributed to tobacco use in 2006 were due to lung cancer.7 The estimated incidence of lung cancer in Spain in 2014 was 22,455 cases in men and 5404 in women, with a rate adjusted for the world population of 51.7 and 11.8 per 100,000 men and women, respectively.8
Smoking trends over time can be a useful predictor of lung cancer incidence and mortality.9 On the other hand, the long period between starting smoking and developing lung cancer makes it a poor indicator for evaluating the outcome of current tobacco control policies: even if the prevalence of smoking falls, mortality due to lung cancer may continue to rise, due to the effects of previous tobacco consumption.
Starting smoking and continuing the habit differs between sexes and ages. In Spain, gender differences have been observed in the prevalence of tobacco use: while it is always more common among men, rates among men have remained unchanged or even fallen in recent years, while smoking has increased among women.10 The Gender Inequality Index (GII) is a tool which measures loss in potential development due to inequality between men and women, according to 3 dimensions: reproductive health, empowerment, and the labor market. Between 1960 and 2010, a close correlation (r=−0.99) was recorded between the GII and the ratio of female smokers to male smokers; as the GII fell, the male/female smoking ratio came close to 1.11 The association between tobacco use by sex and gender inequality, and the fact that gender equality is improving in Spain, may signal an increase in the incidence of tobacco-related cancers, particularly lung cancer, among women, with the accompanying increase in mortality.
These differences in the prevalence of tobacco use by sex underline the importance of examining lung cancer mortality in Spain in recent decades. The objective of this study was to describe and compare gender-related trends in lung cancer mortality in Spain between 1980 and 2013.
MethodsDeath records were retrieved from the National Institute of Statistics12 for the study period (1980–2013) and stratified by sex, age group (18 groups, from 0 to 4 years of age to 85 years or older), and principal cause of death coded according to the 9th and 10th Editions of the International Classification of Disease (ICD),13 depending on the year of the data (ICD-9 from 1980 to 1998 and ICD-10 from 1999 to 2013). For this study, the codes for lung cancer in both editions were used (code 162 for the ICD-9 and codes C33 and C34 for the ICD-10). To calculate the population over the study period, census data were used for 1981, 1991, 2001 and 2011, and estimates from the National Institute of Statistics were used for the intercensus years.12
The crude mortality rate (CMR), the truncated mortality rate (TMR) (from 45 to 74 years of age), direct standard world population for the period 2000-2025 age-adjusted mortality (AAMR)14 and age-specific mortality rates (for the following groups: <45 years, 45–54 years, 55–64 years, 65–74 years, and >=75 years). All rates are presented per 100,000 person-years.
The joinpoint regression model was used to estimate changes in trends in the different mortality rates.15 Years in which the trend changed were identified (joinpoints) and the trend before the first joinpoint, between 2 joinpoints and after the last joinpoint were estimated using the annual percent change (APC) of the rates. The model was limited to a maximum of 2 joinpoints, which would represent 3 different trends. All analyses were performed using the Joinpoint Regression Program, version 4.0.16
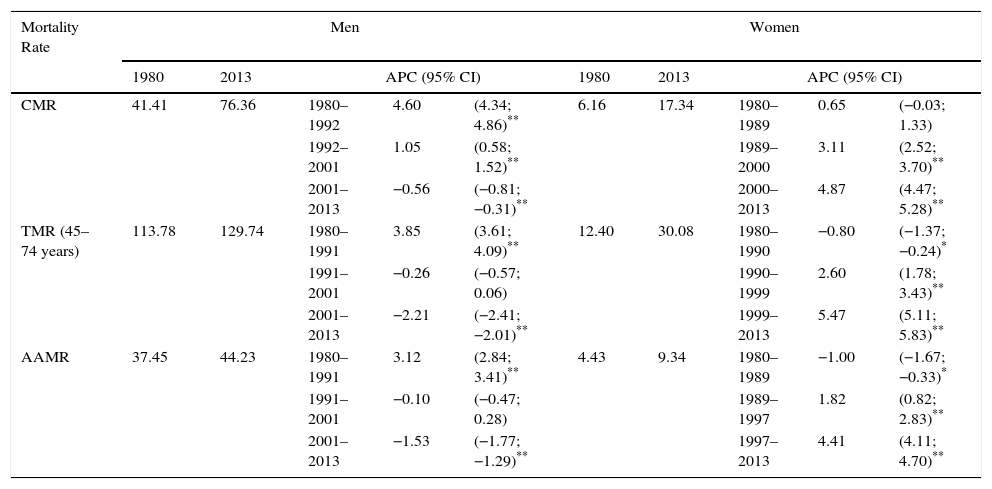
ResultsTable 1 shows the CMR, the TMR and the AAMR for men and women. In men, 3 time trends were observed for CMR, TMR, and AAMR. The CMR showed an increasing trend until 2001, followed by a statistically significant fall (APC=−0.56%). The TMR (age groups 45–74 years) tended to fall after 1992, as did the AAMR. In women, 3 time trends were also observed for CMR, TMR and AAMR; however, in this case, an increase was observed in all rates after the beginning of the 1990s (Table 1), and after the end of 1990s, the increases in TMR and AAMR of around 5% were statistically significant (TMR 5.42% from 1999 and AAMR 4.41% from 1997).
Crude, Truncated and Age-Adjusted Mortality Rates for Lung Cancer and Joinpoint Analysis by Sex in Spain (1980–2013).
| Mortality Rate | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1980 | 2013 | APC (95% CI) | 1980 | 2013 | APC (95% CI) | |||||
| CMR | 41.41 | 76.36 | 1980–1992 | 4.60 | (4.34; 4.86)** | 6.16 | 17.34 | 1980–1989 | 0.65 | (−0.03; 1.33) |
| 1992–2001 | 1.05 | (0.58; 1.52)** | 1989–2000 | 3.11 | (2.52; 3.70)** | |||||
| 2001–2013 | −0.56 | (−0.81; −0.31)** | 2000–2013 | 4.87 | (4.47; 5.28)** | |||||
| TMR (45–74 years) | 113.78 | 129.74 | 1980–1991 | 3.85 | (3.61; 4.09)** | 12.40 | 30.08 | 1980–1990 | −0.80 | (−1.37; −0.24)* |
| 1991–2001 | −0.26 | (−0.57; 0.06) | 1990–1999 | 2.60 | (1.78; 3.43)** | |||||
| 2001–2013 | −2.21 | (−2.41; −2.01)** | 1999–2013 | 5.47 | (5.11; 5.83)** | |||||
| AAMR | 37.45 | 44.23 | 1980–1991 | 3.12 | (2.84; 3.41)** | 4.43 | 9.34 | 1980–1989 | −1.00 | (−1.67; −0.33)* |
| 1991–2001 | −0.10 | (−0.47; 0.28) | 1989–1997 | 1.82 | (0.82; 2.83)** | |||||
| 2001–2013 | −1.53 | (−1.77; −1.29)** | 1997–2013 | 4.41 | (4.11; 4.70)** | |||||
APC, annual percentage change; 95% CI, 95% confidence interval; AAMR, age-adjusted mortality rate; CMR, crude mortality rate; TMR, truncated mortality rate.
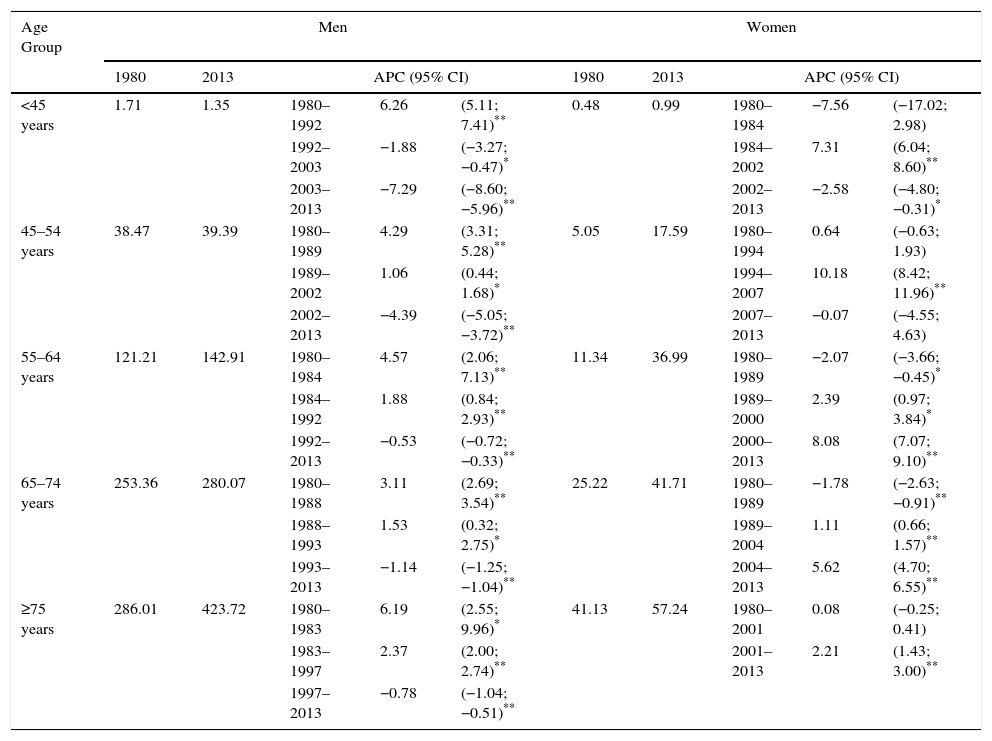
Time trend analysis of lung cancer mortality rates by age groups is given in Table 2. A decreasing trend is observed in men from the mid-1990s, except for the 45–54 age group, which only began to fall as of 2002. The decrease is greater among younger men (<45 years: −7.29% after 2003; 45–54 years: 4.39% after 2002) and lower among the elderly (>75 years: −0.78% after 1997; 65–74 years: −1.14% after 1993). Among women aged <55 years, mortality rates from lung cancer increased over a period of time and then began to level out during the period 2002–2007 (APC=7.31% between 1984 and 2002 in women <45 years, and APC=10.18% between 1994 and 2007 in women aged 45–54 years, Table 2). Since the end of the 1980s approximately, the lung cancer mortality rate rose significantly among women ≥55 years, with a maximum increase after 2000 (APC=8.08% between 2000 and 2013 in women aged 55–64 years; APC=5.62% between 2004 and 2013 in women aged 65–74 years; APC=2.21% between 2001 and 2013 in women aged ≥75 years).
Specific Mortality Rates for Lung Cancer and Joinpoint Analysis by Sex in Spain (1980–2013).
| Age Group | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1980 | 2013 | APC (95% CI) | 1980 | 2013 | APC (95% CI) | |||||
| <45 years | 1.71 | 1.35 | 1980–1992 | 6.26 | (5.11; 7.41)** | 0.48 | 0.99 | 1980–1984 | −7.56 | (−17.02; 2.98) |
| 1992–2003 | −1.88 | (−3.27; −0.47)* | 1984–2002 | 7.31 | (6.04; 8.60)** | |||||
| 2003–2013 | −7.29 | (−8.60; −5.96)** | 2002–2013 | −2.58 | (−4.80; −0.31)* | |||||
| 45–54 years | 38.47 | 39.39 | 1980–1989 | 4.29 | (3.31; 5.28)** | 5.05 | 17.59 | 1980–1994 | 0.64 | (−0.63; 1.93) |
| 1989–2002 | 1.06 | (0.44; 1.68)* | 1994–2007 | 10.18 | (8.42; 11.96)** | |||||
| 2002–2013 | −4.39 | (−5.05; −3.72)** | 2007–2013 | −0.07 | (−4.55; 4.63) | |||||
| 55–64 years | 121.21 | 142.91 | 1980–1984 | 4.57 | (2.06; 7.13)** | 11.34 | 36.99 | 1980–1989 | −2.07 | (−3.66; −0.45)* |
| 1984–1992 | 1.88 | (0.84; 2.93)** | 1989–2000 | 2.39 | (0.97; 3.84)* | |||||
| 1992–2013 | −0.53 | (−0.72; −0.33)** | 2000–2013 | 8.08 | (7.07; 9.10)** | |||||
| 65–74 years | 253.36 | 280.07 | 1980–1988 | 3.11 | (2.69; 3.54)** | 25.22 | 41.71 | 1980–1989 | −1.78 | (−2.63; −0.91)** |
| 1988–1993 | 1.53 | (0.32; 2.75)* | 1989–2004 | 1.11 | (0.66; 1.57)** | |||||
| 1993–2013 | −1.14 | (−1.25; −1.04)** | 2004–2013 | 5.62 | (4.70; 6.55)** | |||||
| ≥75 years | 286.01 | 423.72 | 1980–1983 | 6.19 | (2.55; 9.96)* | 41.13 | 57.24 | 1980–2001 | 0.08 | (−0.25; 0.41) |
| 1983–1997 | 2.37 | (2.00; 2.74)** | 2001–2013 | 2.21 | (1.43; 3.00)** | |||||
| 1997–2013 | −0.78 | (−1.04; −0.51)** | ||||||||
APC, annual percentage change; 95% CI, 95% confidence interval.
Our data show differing, almost opposed, trends in lung cancer mortality according to sex among the Spanish population. Although lung cancer mortality is still higher in men than in women, since the 1990s the rate has fallen in men, but has increased in women. A similar gender pattern has been observed in the incidence of lung cancer in some countries in northern and eastern Europe.17 The only study conducted in Spain which recently compared cancer incidence and mortality data in the region of Catalonia showed similar results for lung cancer until 2007.18 More specifically, lung cancer mortality in men was about 7 times higher at the beginning of 2000, and around 4 times higher 10 years later.17,19,20
Smoking is the major cause of lung cancer.21 For this reason, differences in the gender pattern seen in our study may be explained by changes in the smoking epidemic in Spain. Although more men smoke than women, the gap has narrowed in recent years: in 1995, the prevalence of male smokers was double that of female smokers (48.9% vs 22.5%),22 but the latest Spanish National Health Survey in 2011–2012 showed smaller differences in prevalence between the sexes (27.9% in men and 20.2% in women).23 Lung cancer mortality rates may have increased in women in more recent years because 40 years have passed since the 1970s, when the number of women smoking started to increase: the prevalence rose from 5.8% in 1970 to 15.0% in 1980, and up to 26% in 1990, when figures stabilized, until they began to fall in 2000.22,24 For this reason, since 2001 the percentage of deaths attributable to smoking has been falling in men and rising in women in Spain.7 These results coincide with the pattern of tobacco use in southern Europe, where it is a relatively recent phenomenon among women, while in northern Europe, the prevalence of female smokers peaked many years previously.25–27
Improvements in diagnosis and treatments and less workplace and environmental contamination may also have affected the trend in lung cancer incidence and mortality. However, unlike smoking, these factors have impacted men and women equally, so they do not help explain the differences.
Accordingly, in view of the strong association between gender inequality and smoking habits,11 it seems justified to at least propose a relationship between gender and lung cancer mortality. At the time when gender inequality decreased, smoking rates among men and women became more similar. The tobacco industry even promoted the image of a woman smoking as a symbol of female empowerment.28 Hence, while we did not perform an analysis by gender, we posit that differences between men and women in lung cancer mortality may be associated with gender differences. In order to test this hypothesis of gender-related differences in lung cancer mortality, we would need to have collected sociodemographic variables (educational level, occupation, social class, etc.) or used instruments to accurately measure the role of gender, such as the GII. However, no sociodemographic variables or instruments for measuring gender role were included in the analysis of this study, so the scope of the study for associating differences in lung cancer mortality trends between men and women with gender is limited. More studies are needed to confirm this hypothesis.
It is difficult to pinpoint the moment when mortality rates among women will peak and begin to level out. Our data show that all the rates that we examined have increased in recent years (with the exception of women younger than 45 years). The prevalence of tobacco use is falling slightly, according to the results reported by the Spanish National Health Survey (from 22.5% in 1995 to 20.2% in 2012), but the 30- to 40-year lapse between tobacco consumption and death from lung cancer may explain why lung cancer mortality continues to grow for many more years. Indeed, all registries show that the incidence is continuing to grow.19 Studies performed in countries such as Australia, Netherlands, United Kingdom and the USA show that the percentages of lung cancer deaths attributable to smoking in men and women are converging.29
Since the 1980s, breast cancer has been the main cause of cancer death among women. Figures peaked at the beginning of the 90s and have fallen since the middle of that decade at a rate of 1.8% a year. This fall is attributed to the combined effect of therapeutic advances and early treatment, the latter abetted by the implementation of breast cancer screening programs among the population.30 It is interesting to note that the target age group for breast cancer screening (50–64 years) is the same as the age group in which lung cancer mortality rose in our study – we observed a rise in rates after the age of about 50 years. We would, then, recommend that breast cancer screening programs include campaigns promoting smoking cessation among women smokers invited to participate in screening, in order to reduce the risk of lung cancer. Some studies31–33 have described the phenomenon of hardening among smokers, suggesting that while society is becoming more disapproving of smoking, the remaining smokers show greater nicotine dependence and lower motivation for stopping smoking. However, there is no strong evidence that remaining smokers really do become hardened.34,35 If the hardening hypothesis is correct, then female smokers invited to breast cancer screening may be more reluctant to stop smoking, and accordingly, may need more help to quit. Some subjects may need pharmacological intervention or nicotine replacement therapy36 to help beat physical nicotine dependence.
A limitation of studies based on death certificates is that they may be influenced by the quality of the death records, including problems of data validity and reliability. Nevertheless, some studies have shown the quality of Spanish death records is high, and that these registries are reliable, particularly with regard to the main causes of death, such as cancer, and the major types of cancer, such as lung cancer.37,38 Another problem related with death certificates is that during the study period, 2 different classification systems, ICD-9 and ICD-10, were used. In the case of lung cancer, a concordance of over 98% has been reported between the 2 systems in the number of deaths recorded.39 Moreover, the death registry is complete and covers the whole country. One inherent weakness in our study may derive from the use of lung cancer mortality as an indicator of the presence of disease, due to the high fatality rate of this cancer. A better approach may be to collect disease incidence data, in order to also estimate the morbidity burden of this disease. Another limitation of the study is that the age distribution of the population changed over the study period, particularly due to the large-scale migratory movements of recent years. However, this potential bias was minimized by the use of adjusted and truncated rates.
In conclusion, our study shows gender differences in lung cancer mortality trends in Spain. These differences may be explained by the increased use of tobacco among women in recent years, and the decreased use among men. Smoking cessation must be promoted among women smokers who participate in breast cancer screening programs in Spain.
Authors’ ContributionJMMS designed the study. JCMS prepared the database and performed the analysis. JCMS, JMMS and RC reviewed the results. All authors (JCMS, RC, CLM, LGP and JMMS) contributed to the interpretation of the results. JCMS wrote the first draft of the manuscript, and JMMS performed a critical review. All authors (JCMS, RC, CLM, LGP and JMMS) made a significant contribution to the subsequent versions and approved the final version of the manuscript.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Martín-Sánchez JC, Clèries R, Lidón-Moyano C, González-de Paz L, Martínez-Sánchez JM. Diferencias entre hombres y mujeres en la tendencia temporal de la mortalidad por cáncer de pulmón en España (1980–2013). Arch Bronconeumol. 2016;52:316–320.