Farmer's lung disease (FLD) is a form of hypersensitivity pneumonitis (HP) caused by inhaling microorganisms from hay or grain stored in conditions of high humidity in the agricultural workplace. It is probably underdiagnosed, especially in northern Spain, where climatic conditions favor the development of this disease.
According to previous studies, the most common antigens are usually thermophilic actinomycetes and fungi. The epidemiology of the disease is not well known, and is based on studies conducted by Central European and Asian groups.
The clinical presentation may vary, differentiating the chronic (exposure to lower concentrations of the antigen over a longer period time) and the acute forms (after exposure to high concentrations of the antigen). In patients with respiratory symptoms and agricultural occupational exposure, radiological, lung function and/or anatomical pathology findings must be compatible with FLD, bronchoalveolar lavage must show lymphocytosis, and tests must find sensitivity to the antigen.
The main treatment is avoidance of the antigen, so it is essential to educate patients on preventive measures. To date, no controlled studies have assessed the role of immunosuppressive therapy in this disease. Corticosteroid treatment has only been shown to accelerate resolution of the acute forms, but there is no evidence that it is effective in preventing disease progression in the long-term or reducing mortality.
La enfermedad del pulmón de granjero (EPG) es una forma de neumonitis por hipersensibilidad (NH) producida por la inhalación de microorganismos procedentes del heno o grano almacenado en condiciones de alta humedad en el ámbito laboral agrícola. Se trata de una enfermedad probablemente infradiagnosticada, sobre todo en el Norte de España, donde las condiciones climáticas son propicias para el desarrollo de la misma.
Según estudios previos los antígenos más frecuentes suelen ser hongos y actinomicetos termofílicos. La epidemiología de la enfermedad no es del todo bien conocida, y se basa en estudios realizados por grupos centroeuropeos y asiáticos.
La presentación clínica puede ser variada, diferenciándose las formas agudas (tras exposición a elevadas concentraciones del antígeno) y las crónicas (exposición a menores concentraciones del antígeno, pero más prolongada en el tiempo). En estos casos es esencial, en aquellos pacientes con clínica respiratoria durante la exposición laboral agrícola, demostrar una radiología y función pulmonar compatible, así como una sensibilización al antígeno, una linfocitosis en el lavado broncoalveolar en su caso y/o una anatomía patológica concordante.
El tratamiento principal es la evitación antigénica, por lo que la educación de los pacientes en las medidas preventivas es fundamental. Por el momento, no existen estudios controlados que permitan evaluar el papel de tratamientos inmunosupresores en esta enfermedad. El tratamiento con corticosteroides solo ha demostrado acelerar la resolución de las formas agudas, pero no hay estudios que demuestren su efectividad a largo plazo, con el fin de evitar la progresión de la enfermedad ni disminuir su mortalidad.
Farmer's lung disease (FLD), first described by Campbell in 1932, is one of the most prevalent forms of hypersensitivity pneumonitis (HP).1 It is caused by the inhalation of microorganisms from hay and the dust from grain or straw stored in very damp conditions. It is a significant cause of morbidity among farm workers in some countries.2,3 Few studies have been published in Spain,4–6 in contrast to the large series published in other regions with similar climatic conditions to northern Spain.
EpidemiologyThe exact prevalence of FLD is difficult to determine, since the disease is influenced by many factors, including climate, geographical region, local customs, and differences in the nature and intensity of exposure to antigens.7 Approximately 0.5%–3% of farmers may develop FLD, and the disease is associated with higher mortality rates. More recent studies performed among farmers in Asia described a prevalence of less than 6%,7,8 but few epidemiological studies have been performed in our setting.9
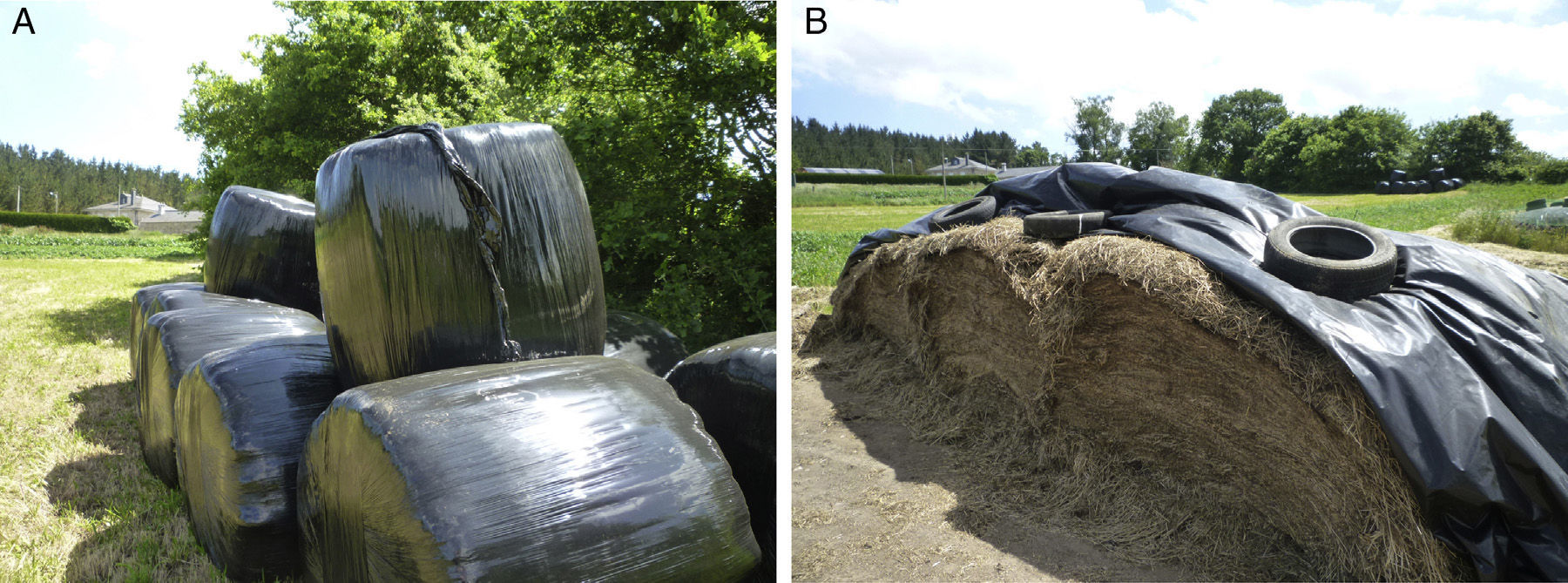
EtiologyThe causative antigens in most cases of HP, including FLD, are bacteria, the most commonly described being thermophilic actinomycetes. This species includes Saccharopolyspora rectivirgula (previously known as Micropolyspora faeni, described as the main FLD antigen), Thermoactinomyces vulgaris, Thermoactinomyces viridis, and Thermoactinomyces sacchari, among others. These organisms reproduce in areas with high levels of humidity and at temperatures of between 40 and 60°C. They are often isolated in contaminated farms (from moldy hay and other types of fodder), milking sheds and compost plants. These bacteria are among most common etiological agents in studies reported in Europe and North America,10,11 but farmers are also exposed to other fungi and fungal fragments that can also cause FLD, such as Alternaria, Aspergillus fumigatus and Botrytis.11,12 FLD, then, is common in farming areas, particularly in the cold, wet seasons, when climatic conditions lead to increased levels of microorganisms in stored hay (Fig. 1).12
PathogenesisAll HP diseases, including FLD, are caused by repeated exposure to antigenic particles in a susceptible, previously sensitized patient. The characteristics of antigens that determine their ability to induce an immunological response include their size, solubility, nature, resistance to enzymatic degradation, and inflammatory capacity. These antigens are implicated in the formation of antigen-antibody immune complexes, particularly of the IgG type, which intervene in complement activation. Antibody response alone is insufficient to cause disease: a cytotoxic CD8+ lymphocyte response is also required. Lymphocyte mediation is another mechanism involved in the process, particularly Th1 mediation, which is responsible for lymphocyte alveolitis and the formation of granulomas.1,13 Some studies have shown that lymphocytes are also involved in the pathogenesis of FLD, and evidence of lymphocyte stimulation is considered as diagnostic proof of the disease.14 Immediate hypersensitivity reactions, probably caused by IgG4 rather than IgE, may also play a role in the genesis of the immunological response.13,15
Little information is available on the particular host characteristics which determine susceptibility to developing FLD. It is more common in middle-aged men, although this probably reflects differences in exposure levels. It is also more common in non-smokers, probably because tobacco reduces the IgG response to inhaled antigens, affects cytokine production, and alters macrophage function.16,17
Known environmental risk factors include antigen load, duration of exposure, rhythm of exposure (frequency/intermittency), use or non-use of respiratory protection, and the type of working practices.11
Clinical FormsFLD is conventionally classified into 3 groups (acute, subacute, and chronic), depending mainly on clinical and radiological findings at the time of diagnosis.18
AcuteAcute disease occurs after exposure to high concentrations of antigen over a short period. Symptoms appear 4–8h after exposure, and tend to resolve quickly. It is characterized by non-specific symptoms, such as general malaise, low-grade fever or fever, and dry cough. The most severe cases present rapidly progressing dyspnea. Physical examination reveals fine crackles on pulmonary auscultation.
SubacuteSubacute disease occurs after continuous, but not massive, inhalation of antigens. Symptoms develop more insidiously. It is characterized by general malaise, low-grade fever, asthenia and anorexia, progressive development of dyspnea and non-productive cough.
ChronicChronic disease occurs after exposure to lower antigen levels, but over longer periods. It is also described as a progression of untreated acute or subacute disease. It usually occurs with symptoms of progressive dyspnea on exertion and dry cough. Physical examination reveals digital clubbing and dry crackles on auscultation. Chronic obstructive pulmonary disease with centrilobular emphysema, rather than fibrosis, has been described in patients with recurrent acute episodes.19
This classification of patients into 3 groups has frequently been challenged, and in 1 study, the authors preferred to classify FLD as only either acute or chronic. Lacasse et al. analyzed 168 FLD patients, assigning them to 2 groups, according to clinical-radiological findings. The first group, consisting of 41 patients, had recurrent symptoms of wheezing and fever, and no changes on standard chest X-ray. In contrast, the second group, consisting of 127 patients, showed digital clubbing, hypoxemia, restrictive functional respiratory changes, and radiological signs of established fibrosis. The only overlapping feature was ground glass findings on the chest high-resolution computed tomography (HRCT).20
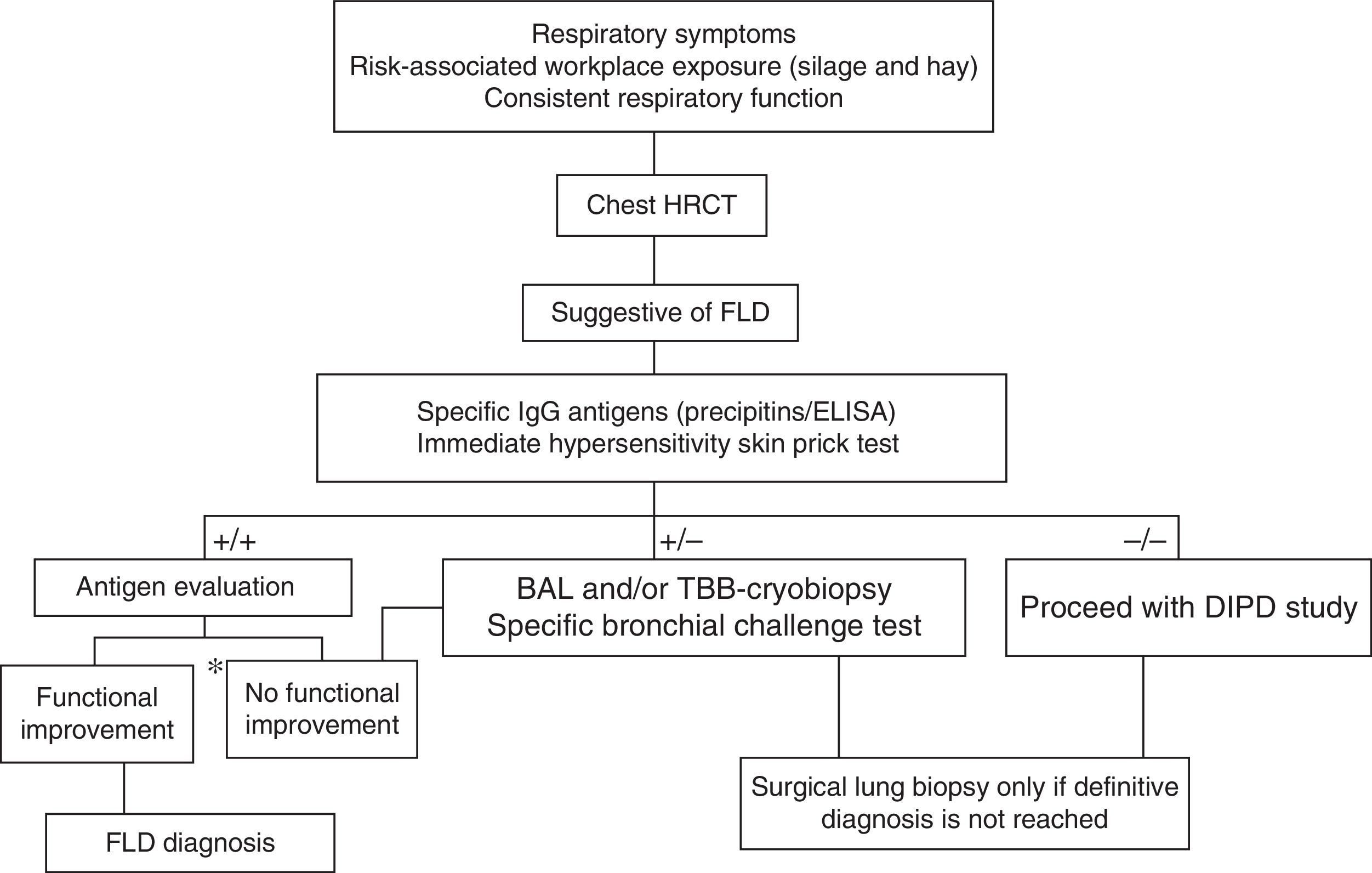
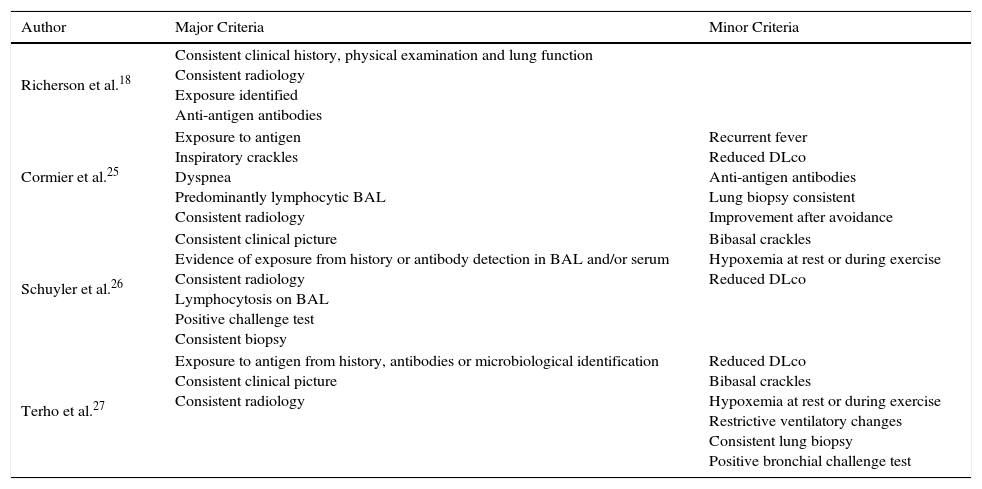
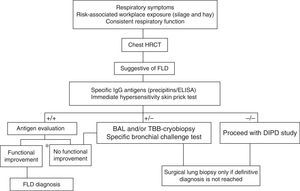
DiagnosisSeveral conventional diagnostic criteria have been proposed for FLD, but none have been validated. The major criteria are summarized in Table 1. Like other diffuse interstitial pulmonary diseases (DIPD), the point of departure is clinical suspicion, backed up by a detailed history of workplace and environmental exposure, suggestive clinical symptoms and lung function values, radiological findings, cytological evidence on bronchoalveolar lavage (BAL), and consistent pathology results21,22 (Fig. 2). The following additional testing is then performed:
Main Criteria Proposed for the Diagnosis of Hypersensitivity Pneumonitis.
| Author | Major Criteria | Minor Criteria |
|---|---|---|
| Richerson et al.18 | Consistent clinical history, physical examination and lung function Consistent radiology Exposure identified Anti-antigen antibodies | |
| Cormier et al.25 | Exposure to antigen Inspiratory crackles Dyspnea Predominantly lymphocytic BAL Consistent radiology | Recurrent fever Reduced DLco Anti-antigen antibodies Lung biopsy consistent Improvement after avoidance |
| Schuyler et al.26 | Consistent clinical picture Evidence of exposure from history or antibody detection in BAL and/or serum Consistent radiology Lymphocytosis on BAL Positive challenge test Consistent biopsy | Bibasal crackles Hypoxemia at rest or during exercise Reduced DLco |
| Terho et al.27 | Exposure to antigen from history, antibodies or microbiological identification Consistent clinical picture Consistent radiology | Reduced DLco Bibasal crackles Hypoxemia at rest or during exercise Restrictive ventilatory changes Consistent lung biopsy Positive bronchial challenge test |
Proposed diagnostic algorithm for farmer's lung disease. BAL: bronchoalveolar lavage; DIPD: diffuse interstitial pulmonary disease; FLD: farmer's lung disease; TBB: transbronchial biopsy. *Respiratory function improvement >20% of FVC, FEV1 and/or DLco. Source: adapted from the algorithm proposed for the diagnosis of HP by Morell et al.28
Specific IgG antibodies or precipitins (precipitating IgG antibodies) against the different suspected antibodies should be determined in the patient's serum, in order to confirm that the individual has been exposed and is sensitized to the causative agent. A negative result for plasma precipitins does not rule out the diagnosis, particularly in the chronic forms of the disease, since antibody titers and levels of exposure are correlated, and may become negative if there has been no contact with the causative antigen for some time. The determination of precipitins may also be limited, firstly by the lack of standardized analytical methods, and secondly because the panel of precipitins will vary among the different regions, so it will be necessary to determine which are the most common antigens in each site.23,24 In this respect, it is also very useful to perform a culture for fungi and actinomycetes from the hay or other material to which the patient has been exposed.
Since it may be difficult to detect specific antibodies in certain situations, other methods have been proposed for evaluating sensitization. Morell et al.13 studied the diagnostic yield of the leukocyte migration inhibition test (LMIT) in 20 patients with FLD and in 24 asymptomatic farmers. In the first group the test was positive in 95% (19/20), while it was positive in only 44% (11/25) of the control group: the difference was statistically significant (P<.005). The LMIT was also studied in 8 FLD patients who had had no contact with the antigen during the previous year: the test was positive in 87% (7/8). The authors concluded that the test was even more effective than specific antibody testing for diagnosing FLD, even in patients who had had no contact with the antigen in the previous year.
Other laboratory parameters are unspecific. Leukocytosis, raised immunoglobulin subgroups (IgG, IgM and IgA), and C-reactive protein are seen in the acute forms.
Skin Prick TestsSkin prick tests for delayed sensitivity to antigens are considered very unspecific, but some studies have demonstrated diagnostic benefit, particularly when read immediately (10–15min), both in FLD, with a sensitivity of 83% and a specificity of 72%,29 and in bird fancier's lung, with a sensitivity of 90% and a specificity of 85%.30
Bronchial Challenge TestingSpecific bronchial challenge testing with the antigen in question is highly sensitive and specific (85% and 86%, respectively) for evaluating patients exposed to bird and fungal antigens. These tests aim to reproduce the clinical symptoms and effects on lung function that would occur in the working environment. It is a test which must be performed in the hospital setting, under medical supervision, and not all centers have the right facilities.31
RadiologyIn acute phases, the chest X-ray can be normal or show diffuse pulmonary infiltrates. In the chronic phases, a bilateral reticular pattern may be observed.
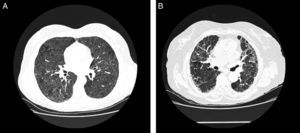
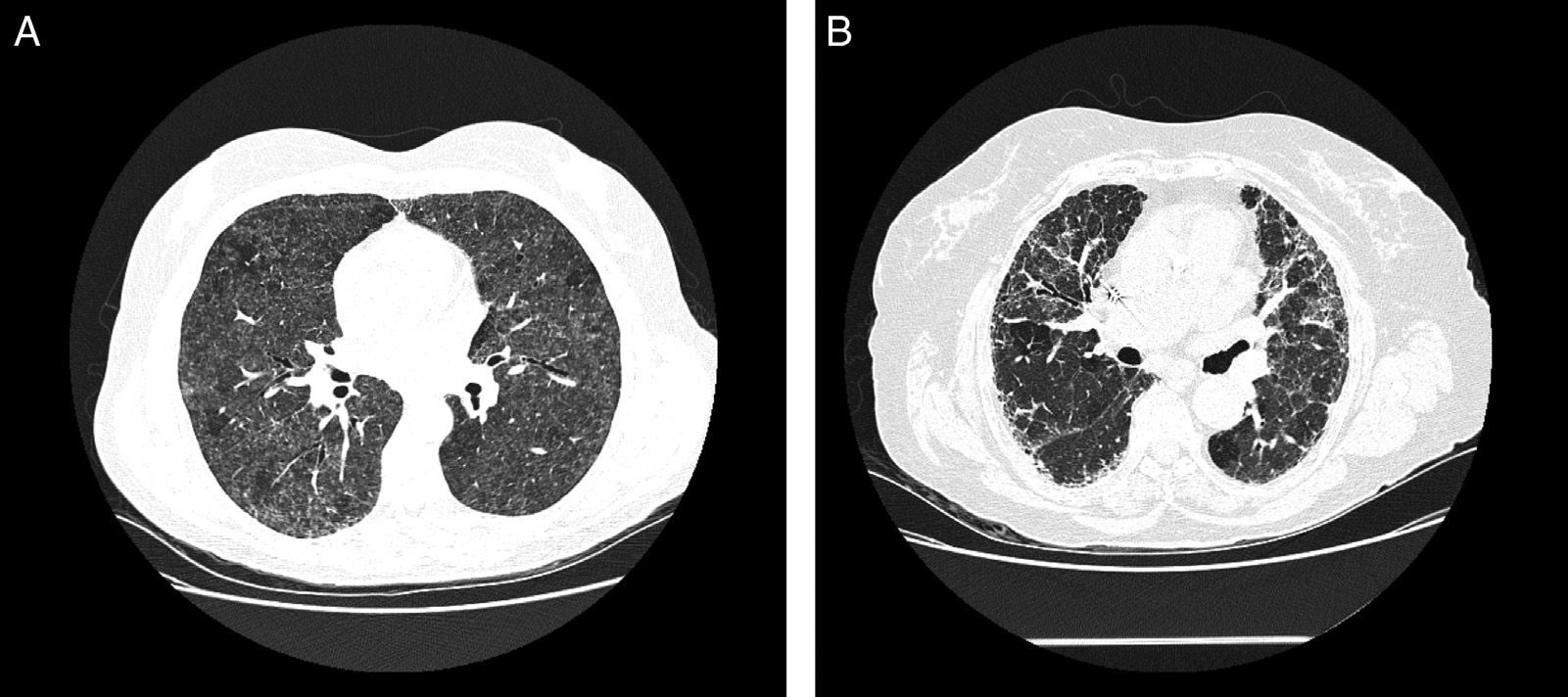
In acute phases, typical chest HCRT findings are diffuse ground glass infiltrates and hyperlucent images (as a result of concomitant bronchiolitis), which together form a pattern of mosaic attenuation.32 Centrilobular nodules are also typical in some disease phases, but they are more common in the acute phases32 (Fig. 3A). Honeycombing is an important feature of chronic forms. Emphysema is observed in 20% of non-smokers with FLD.33,34
Radiological characteristics in farmer's lung disease. (A) Chest high-resolution computed tomography in a patient with acute farmer's lung. Ground glass infiltrates and centrilobular nodules can be observed. (B) Chest high-resolution computed tomography in a patient with chronic farmer's lung. Note the reticular pattern in middle fields with low-grade ground glass infiltrate.
Involvement is typically limited to the middle and upper lung fields, although lower fields cannot be ruled out, which means that at times disease appears on CT primarily in a honeycombing pattern and the radiological image is indistinguishable from that of usual interstitial pneumonia (UIP)35 (Fig. 3B).
Respiratory FunctionPatients have a restrictive ventilatory pattern, with altered gas exchange (reduced DLco and desaturation on exertion), also observed with other DIPDs. In chronic forms of FLD, an obstructive ventilatory pattern may occur along with emphysema.36–38
Specific Inhalation ChallengeIn unclear cases, the diagnosis must be confirmed with a specific inhalation challenge (SIC) conducted with suspected antigens – a range of molds and/or actinomycetes, depending on the region. SIC can now be considered as validated, after the publication of studies in other types of HP, particularly in bird fancier's lung,39–41 and the more recent paper from Muñoz et al.,31 in both bird fancier's lung and HP. Thus, before performing a surgical lung biopsy, an SIC must be conducted with the appropriate antigens, in hospitals where it is available.
BronchoscopyIn the study of FLD and other types of DPID, BAL can be used for guiding the differential diagnosis. BAL cytology in FLD generally reveals raised CD8 lymphocyte levels (lymphocyte predominance >20% and usually, but not always, an inverted CD4/CD8 lymphocyte ratio).42
Transbronchial Biopsy, Cryobiopsy, and Surgical Lung BiopsyTransbronchial biopsy (TBB) can be useful in the early stages of the disease.43 TBB by cryoprobe (or cryobiopsy) is a less invasive endoscopic technique than surgical lung biopsy, and may play an important role in the diagnosis of FLD. Recently published studies report a diagnostic yield in DPID of over 70%, with relatively few complications.44–46
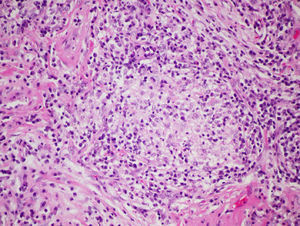
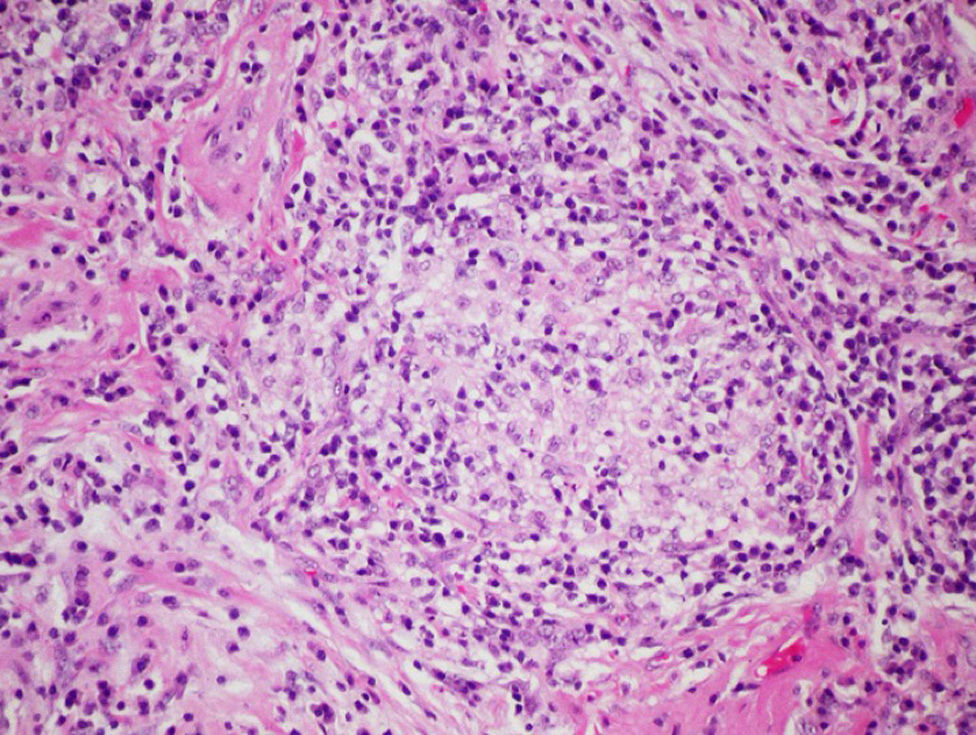
Surgical lung biopsy is not usually required to establish a diagnosis of FLD, but it is often vital in differentiating a chronic form of FLD from other interstitial entities, particularly in cases in which no antigen can be identified.28,47 In general, it is rarely required in the acute phases, since the causal relationship is usually easy to establish. The largest series published to date is that of Hariri et al., who described 5 cases of acute HP, although the findings suggest that these may be acute exacerbations of a previous HP, rather than an acute HP as such, since intra-alveolar fibrin with some interstitial eosinophils and neutrophils that occur along with subacute changes, such as lymphocytic bronchiolitis and loose, non-necrotizing granulomas, were observed.48 For this reason, histological findings in the acute phase of the disease are still not fully clarified. Alveolar infiltrates with neutrophils, eosinophils and necrotizing vasculitis of the small vessels have been described.48 In the subacute and chronic forms of the disease, the relationship between the onset of symptoms and causal exposure is less clear, and fibrosing changes may occur in some cases, suggestive of a pattern of usual interstitial pneumonia (UIP) or non-specific interstitial pneumonia (NSIP).49 In general, the typical histological findings (but not the pathognomic findings) are reflected in the conventional triad described by Coleman and Colby,50 which have been described in more detail in some more recent articles, namely: (1) predominantly lymphocytic interstitial pneumonia; (2) chronic bronchiolitis in the form of predominantly lymphocytic peribronchiolar infiltrate with or without fibrosis; and (3) predominantly interstitial, loose, non-necrotizing granulomas51 (Fig. 4). However, it should be taken into account that granulomatous findings may be absent in up to 30% of cases. Some publications even report that the complete triad exists only in 50% of the cases,48 and the presence of peribronchiolar metaplasia has also been described.49
Differential DiagnosisThe differential diagnosis is performed basically against other DIPDs. Chronic forms may be similar to idiopathic pulmonary fibrosis (IPF) and fibrotic phase NSIP, with a progressive clinical picture and few symptoms. Even if a surgical lung biopsy is available, differential diagnoses suggested by that specimen can include other entities, ranging as widely as sarcoidosis, drug-induced DIPDs, lymphocytic interstitial pneumonia, and DIPDs due to connective tissue diseases. In advanced chronic forms, it may be difficult to differentiate the histological pattern of UIP from IPF, or from the NSIP pattern, although fibrosis spreading toward the center of the lobule in biopsies with a UIP pattern can indicate the possibility of chronic HP.50–52 Autopsy series have found that fibrosis is more common than granulomas in the chronic forms of the disease.17
The initial lack of specific symptoms in the acute forms may initially suggest an influenza-like process in cases with normal standard chest X-ray, or bacterial pneumonia in patients with pulmonary infiltrates.
Hence, while the most common features can guide diagnosis, the wide range of clinical, radiological and histological presentations require us to establish a strict correlation between them all before reaching a definitive diagnosis.
TreatmentThe treatment of FLD is based mainly on avoiding exposure to the antigen: we will address this approach in more detail in the section on prevention. This is the only measure that has been shown to delay disease progression.
Treatment with glucocorticosteroids accelerates recovery in the acute forms, but it has not been shown to affect the progress of the disease in the long term, and some authors question their role in the chronic forms.13,53 Corticosteroids are traditionally recommended in patients with impaired lung function. The doses are similar to those given for other DIPDs, such as sarcoidosis (30mg/day for 1 month, then 20mg/day, followed by tapering to a maintenance dose of 7.5–15mg/day). No scientific evidence is available for recommending either the use or avoidance of inhaled glucocorticosteroids.
Immunosuppressive treatments have been tested in refractory forms of other HPs, such as bird fancier's lung, but not in FLD. In these patients, the few published cases have reported improved functional progress with rituximab.54,55
Lung transplantation is indicated in cases of progressive disease with respiratory failure, despite the treatments mentioned above. A recent study found improved 5-year survival in patients transplanted due to HP, compared to those transplanted for IPF.56
PreventionThere are 3 ways of reducing repeated inhalation of organic particles by FLD patients, in order to prevent the disease progressing to a fibrosing, and hence irreversible, process: (a) definitive withdrawal of the patient from the farming environment, which in most cases is unfeasible for financial reasons; (b) development of new techniques for drying the hay, and ventilation and mechanization of stock feeding; and (c) use of respiratory protective equipment to prevent the antigens entering the respiratory tree.
One of the main preventive measures consists of improving hay storage methods. Drying or heating the fodder during storage has proven to be beneficial in reducing antigen levels.57 Keeping the fodder in loosely packed square bales also hinders the growth microorganisms, but these methods are disappearing, due to the high cost and heavy labor burden, and are being replaced by round bales (Fig. 1). These bales are easy to store and handle, but they contain high humidity levels due to greater compaction of the hay.58 It is also important that silos are emptied more slowly to reduce the amount of dust in the air (and thus the antigen levels), and the time of exposure. Moreover, special care must be taken to keep the storage space well ventilated, with the creation of continuous flow systems to reduce CO2 and bioaersol levels.59 However, these installations are costly, making widespread implementation unlikely.
In some regions, traditional practices, such as salting the hay, are employed for preventing the proliferation of microorganisms, but these are ineffective.58–60 Commercially available additives can also be applied to the fodder to improve storage conditions, but they tend to be very corrosive acids which damage farm machinery, so arable and pastoral farmers are reluctant to use them.
The use of protective respiratory equipment is a prevention method which requires no suspension of farming activity (Fig. 5). In 1971, the clinical observations of Gourley and Braidwood61 confirmed the efficacy of protective masks in patients with a diagnosis of acute FLD. The filters used retained particles of 0.8μ with a 98% efficacy. Kusaka et al.62 studied a cohort of 21 patients with a diagnosis of FLD who used protective respiratory devices for a period of 2 years during their usual farming activities, particularly when handling hay. During the study period, a significant reduction in symptoms of acute exposure was observed, compared to symptoms suffered in the previous 4 years, and only 1 patient had severe acute symptoms requiring hospitalization and/or corticosteroid treatment. However, the efficacy of respiratory protection in the prevention of chronic disease has not yet been confirmed in other studies.
Some types of respiratory protection may be uncomfortable to use, leading to poor compliance. In the above-mentioned study, of the 21 patients who received protective respiratory devices, 17 (80%) tolerated them for a period of 2 years; the 4 patients who abandoned them did so due to discomfort and lack of symptoms when working without protective systems.62 Tolerance of protective respiratory devices is probably related with prevention of the acute symptoms triggered by exposure and the fact that the only other alternative is changing jobs. When educating patients in the use of these devices, it is important to (1) select the most comfortable and effective device against the antigen to be avoided, and (2) provide advice on care and maintenance.
PrognosisThe progress of FLD varies widely and depends fundamentally on time and antigen load. Even so, in some patients, the disease continues to progress for as yet unknown reasons, despite the use of appropriate avoidance techniques.
The acute phase of FLD is generally reversible, but continuous exposure or several subacute episodes of hypersensitivity to the allergen can cause fibrotic forms to develop, causing irreversible changes in lung structure and function.36,63 Up to 20% of acute forms progress to chronicity. A Finnish study estimated mean 17-year survival after the appearance of symptoms at 9%–17%.64 Factors associated with greater mortality in FLD are a fibrotic pattern in the chest CT65 and significant pulmonary arterial hypertension.66
ConclusionsIn summary, FLD is a disease that may possibly be underdiagnosed in the north of Spain, although no precise epidemiological studies are available. Data on the long-term efficacy of corticosteroid treatment and other immunosuppressants is also scant, and no regional antigen panels are available for diagnostic purposes. More awareness of a suspected diagnosis is needed, taking into account that presentation may not fulfill the conventional criteria for DIPD (e.g., obstructive ventilatory changes and emphysema on chest HRCT). Patients with a diagnosis of FLD should be encouraged to follow a strict strategy for preventing antigen exposure, including, in particular, the proper use of protective respiratory equipment.
Conflict of InterestsThe authors state that they have no conflict of interests.
Our thanks to the other members of the Galician Multidisciplinary Group for Diffuse Interstitial Pulmonary Diseases (GAMEPID): José Blanco, Ana González, Coral González, Noemí Mengual, Isabel Otero, Cristina Ramos, Carlota Rodríguez and Juan Suárez.
We also thank Dr Antón Penas for sharing the images shown in Fig. 1.
We thank 3M for kindly providing the equipment shown in Fig. 5.
Please cite this article as: Cano-Jiménez E, Acuña A, Botana MI, Hermida T, González MG, Leiro V, et al. Revisión de la enfermedad del pulmón de granjero. Arch Bronconeumol. 2016;52:321–328.




 20% of FVC, FEV1 and/or DLco. Source: adapted from the algorithm proposed for the diagnosis of HP by Morell et al.28' title='Proposed diagnostic algorithm for farmer's lung disease. BAL: bronchoalveolar lavage; DIPD: diffuse interstitial pulmonary disease; FLD: farmer's lung disease; TBB: transbronchial biopsy. *Respiratory function improvement >20% of FVC, FEV1 and/or DLco. Source: adapted from the algorithm proposed for the diagnosis of HP by Morell et al.28'/>
20% of FVC, FEV1 and/or DLco. Source: adapted from the algorithm proposed for the diagnosis of HP by Morell et al.28' title='Proposed diagnostic algorithm for farmer's lung disease. BAL: bronchoalveolar lavage; DIPD: diffuse interstitial pulmonary disease; FLD: farmer's lung disease; TBB: transbronchial biopsy. *Respiratory function improvement >20% of FVC, FEV1 and/or DLco. Source: adapted from the algorithm proposed for the diagnosis of HP by Morell et al.28'/>