Acute or chronic stridor are common in pediatric, laryngomalacia being the most common cause of chronic stridor, usually resolving spontaneously during the second year of life.1 Congenital anomalies of mediastinal great vessels, causing tracheomalacia, are included in the differential diagnosis of persistent stridor in children.2
A 15-month-old full-term male infant presented with a history of shortness of breath and stridor. He had previous repeated episodes of wheezing and breathing difficulty, under inhaled prophylactic treatment, and persistent stridor since 9-months-old.
On examination, intercostal and subcostal retractions were seen, polypnea and hypoxemia that improved with high-flow nasal cannula. A biphasic stridor was noticed. Several café-au-lait spots were visible. Blood tests revealed white blood cell normal count and negative C-reactive protein. Capillary blood gas test was normal. Chest X-ray showed bilateral hyperinflated lungs. Due to the persistent and refractory respiratory insufficiency, he was admitted to pediatric intensive care unit, remaining on non-invasive ventilation for 3 days.
Dermatology and ophthalmology evaluation confirmed 12 café-au-lait spots larger than 5mm, but no other criteria for type I neurofibromatosis (NF-1). Neuro-development was normal.
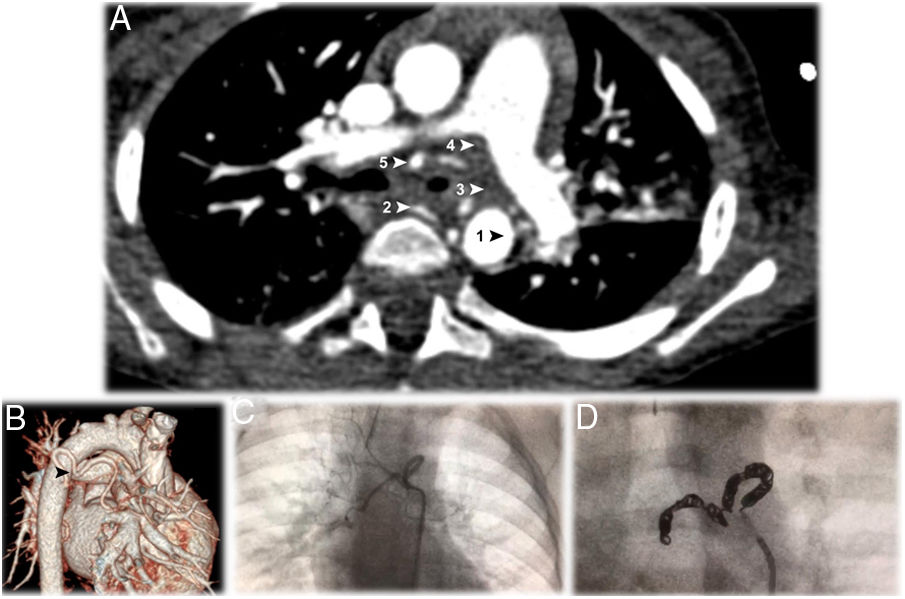
Two months later, he maintained expiratory stridor. Elective bronchofibroscopy revealed lower tracheomalacia and almost total occlusion of left main bronchus (LMB) by pulsatile slit stenosis. A transthoracic echocardiogram revealed normal structure and function of the heart and the large blood vessels. Computed tomography angiography (CTA) detected a vascular anomaly surrounding the LMB (Fig. 1A and B). This anomalous artery originated in the right side of the descending thoracic aorta, from which a normal intercostobronchial branch of normal caliber and a tortuous anomalous bronchial artery originated, dividing into two bronchial arteries, right and left, circled almost all of the LMB, causing its obstruction. None of these affected pulmonary vascularization.
A – Thoracic computed tomography angiography (CTA), in axial plane, showing the anomalous artery, originating in the right side of the descending thoracic aorta (AoD), encircling the left main bronchus at the point of bronchial stenosis. The anomalous bronchial artery originates two bronchial arteries, right and left. The five arrows point to 1: the origin at the aorta; 2: the first branch, which is a normal right intercostobronchial branch; 3: the second branch, which is an aberrant bronchial trunk; 4 and 5: the two vessels originate from the bronchial trunk to form right and left bronchial arteries.
B – Computerized tomography reconstruction arteriography view showing the tortuosity of the anomalous bronchial artery described in a (
).C – Dynamic image showing the anomalous bronchial artery tortuous path around the left main bronchus.
D – Endovascular embolization of the abnormal vessel, occluded with 12 microcoils.
Due to the associated risk with the uniqueness and location of the aberrant bronchial vessel, surgical treatment was not an option. He was treated with endovascular embolization of the abnormal vessel (Fig. 1C), occluded with 12 microcoils (MReye® Embolization Coil, Cook Medical, USA) (Fig. 1D), after selective catheterization of the aberrant bronchial artery (Performa® Mikaelson 4F, Merit Medical, France) and progression into the vessel with a microcatheter (ProGreat® Micro Catheter 2.7 Fr, Terumo, Japan).
Post-procedure bronchoscopy, one month later, showed more than 50% increase in the internal luminal diameter of the LMB. No bronchial prosthesis was required. Six months after treatment, the patient showed no stridor or acute wheezing episodes.
Chronic stridor that does not improve over time, worsens, or is associated with respiratory distress, cyanosis, apnea, dysphagia, aspiration, failure to thrive, or a radiological abnormality, deserves a bronchoscopic evaluation.3,4
Our 15-months-old infant presented several episodes of wheezing in his first months of life, despite prophylaxis, and developed persistent stridor at 9-months-old. He had 12 café-au-lait spots and no other major criteria of NF-1. Bronchoscopy examination found tracheomalacia and LMB stenosis by pulsatile extrinsic compression, leading us to suspect of vascular anomaly or mediastinal mass.
The relatively late installation of stridor and the absence of digestive symptoms were against classical forms of vascular rings.3
Some patients with NF-1 present posterior mediastinal masses (neurofibroma and meningocele) associated with bone injury,5 eventually responsible of stridor and wheezing, due to compression of tracheobronchial tree. Our patient did not present any diagnostic criteria of NF-1, apart from café-au-lait spots,6 his mediastinum was normal and bone lesions were not found.
A normal chest radiography should not exclude vascular or airway anomaly. Bronchoscopy allows determination of site and severity of obstructions and identify endobronchial pathology.7,8 CTA or MRI are procedures of choice for definitive evaluation, allowing surgery planning.8 Echocardiogram should be performed to clarify anatomy, exclude other cardiac anomalies and facilitate intraoperative management. A normal exam does not exclude a vascular malformation and an angiographic examination must be performed.
Our infant had a normal chest X-ray and echocardiogram. Bronchoscopy examination was compatible with vascular compression. CTA excluded mediastinal masses and confirmed the presence of a vascular anomaly that did not fit Klinkhamer and Stewart classification criteria.9,10 We believe that this tracheobronchial vascular malformation was not reported beforehand. Most of the vascular anomalies can be explained by abnormal embryological differentiation of the aortic arch and central pulmonary artery system leading to complex pathologic relationships of these vessels with the tracheobronchial tree.11 Regarding the bronchial arteries, their anatomy of is quite variable in terms of origin and number, originating commonly from the descending thoracic aorta.12
Management of a child with vascular compression involves surgical correction that allows normal tracheal growth and decreases morbidity.13 Embolization of anomalous branches is an alternative, based on other procedures in pediatric age, such as treatment of pulmonary arteriovenous malformation. The difficult surgical approach in this case and non-involvement of pulmonary vascularization helped in the decision.
Endovascular therapy is a minimally invasive procedure, with the goal of effectively treat underlying problem with minimal or no damage to the surrounding structures.14 In this outpatient treatment, the feeding artery of the vascular anomaly is occluded with coils, plugs or a combination of both, with minimal morbidity and no mortality.14 Knowledge of the standard and variant vascular anatomy of the involved territory as well as an understanding the target lesion circulation is essential to achieve safe and effective embolization and avoid complications.
Placement of endo-bronchial stent to enlarge bronchial lumen of a non-compliant structure was considered but not necessary.15 After embolization, clinical and bronchoscopy control was performed, showing therapeutic success, as malacia resolved and there were no further episodes of stridor or wheezing. Tracheobronchial cartilage preserved regenerative capacity after anomalous vessel embolization.
In summary, a high index of suspicion and a prompt, thorough clinical and radiologic evaluation ensures prompt diagnosis and appropriate intervention of congenital vascular anomalies. Bronchoscopy is mandatory to study patients with severe and/or progressive stridor, as well those cases associated with unusual features. CTA is important even in the presence of normal chest radiography and echocardiogram. Endovascular embolization is a minimally invasive technique with very high success, minimal morbidity and no mortality, and should be considered as an alternative to surgery.