Geographical variations may impact outcomes in chronic obstructive pulmonary disease (COPD). We evaluated differences in baseline characteristics and outcomes between patients enrolled in Latin America compared with the rest of the world (RoW) in the TIOtropium Safety and Performance In Respimat® (TIOSPIR®) trial.
MethodsTIOSPIR®, a 2–3-year, randomized, double-blind trial (n=17116; treated set), compared safety and efficacy of once-daily tiotropium Respimat® 5 and 2.5μg with tiotropium HandiHaler® 18μg. This post-hoc analysis pooled data from all treatment arms to assess mortality, exacerbations, cardiac events, and serious adverse events (SAEs) between both regions.
ResultsAt baseline, patients enrolled in Latin America (n=1000) versus RoW (n=16116) were older, with higher pack-years of smoking history and more exacerbations, but less cardiac history. In this analysis, patients in Latin America versus RoW had an increased risk of death (hazard ratio [HR] [95% confidence interval (CI)]: 1.52 [1.24–1.86]; P<.0001) or moderate-to-severe exacerbation (HR [95% CI]: 1.29 [1.18–1.41]; P<.0001), but a lower risk of severe exacerbation (HR [95% CI]: 0.82 [0.68–0.98]; P=.0333). SAE rates in Latin America were lower versus RoW (incidence rate ratio [IRR] [95% CI]: 0.82 [0.72–0.92]), including cardiac disorders (IRR [95% CI]: 0.68 [0.48–0.97]). Risk of major adverse cardiovascular events were similar (HR [95% CI]: 0.99 [0.71–1.40]; P=.9677).
ConclusionsTIOSPIR® patients in Latin America had a higher risk of death or moderate-to-severe exacerbation, but a lower risk of severe exacerbation than those in RoW. Geographical differences may impact outcomes in COPD trials.
Las variaciones geográficas pueden afectar a los resultados en la enfermedad pulmonar obstructiva crónica (EPOC). Evaluamos las diferencias en las características basales y los resultados de los pacientes incluidos en Latinoamérica en comparación con el resto del mundo (RdM) en el ensayo TIOtropium Safety and Performance In Respimat® (TIOSPIR®).
MétodosTIOSPIR®, es un estudio aleatorizado, doble ciego de 2-3 años de duración (n=17.116; conjunto tratado), comparó la seguridad y la eficacia del tiotropio Respimat® una vez al día en dosis de 5 y 2,5μg con respecto al tiotropio HandiHaler® 18μg. Este análisis post-hoc reunió datos de todos los brazos de tratamiento para evaluar la mortalidad, las exacerbaciones, los acontecimientos cardíacos y los acontecimientos adversos graves (AAG) entre ambas regiones.
ResultadosAl inicio del estudio, los pacientes reclutados en América Latina (n=1.000) versus RdM (n=16.116) eran de mayor edad, con más paquetes/año de consumo de tabaco en sus antecedentes y más exacerbaciones, pero menos antecedentes cardíacos. En este análisis, los pacientes de Latinoamérica versus RdM tenían un mayor riesgo de muerte (razón de riesgo [HR] intervalo de confianza del 95% [IC 95%]: 1,52 [1,24-1,86]; p<0,0001) y de exacerbación moderada a grave (HR [IC 95%]: 1,29 [1,18-1,41]; p<0,0001), pero menor riesgo de exacerbación grave (HR [IC 95%]: 0,82 [0,68-0,98]; p=0,0333). Las tasas de AAG en Latinoamérica fueron más bajas frente al RdM (tasa de incidencia [IRR] [IC 95%]: 0,82 [0,72-0,92]), incluidos los trastornos cardíacos (IRR [IC 95%]: 0,68 [0,48-0,97]). El riesgo de acontecimientos cardiovasculares adversos mayores fue similar (HR [IC 95%]: 0,99 [0,71-1,40]; p=0,9677).
ConclusionesLos pacientes de TIOSPIR® en Latinoamérica tuvieron un mayor riesgo de muerte y de exacerbación moderada a grave, pero un menor riesgo de exacerbación grave que aquellos en el RdM. Las diferencias geográficas pueden afectar los resultados en los ensayos de la EPOC.
Chronic obstructive pulmonary disease (COPD) represents an enormous health burden worldwide. Its prevalence is relatively high in Latin America, affecting an estimated 14.3%1 of the population versus 9.2%2 in the rest of the world (RoW). A substantial proportion of Latin American patients had experienced an exacerbation (18.2%),3 and respiratory causes, including COPD, are amongst the leading cause of mortality in this region.4 Smoking is a behaviour that is seemingly ingrained in Latin American culture,5 with smokers from Latin America constituting 8%–10% of tobacco smokers globally.6 Solid fuels are used by 30%–75% of households7 (traditionally in rural areas8) and contribute to mortality rates similar to those observed in tobacco smokers.9 Management guidelines for COPD recognize that curbing the smoking epidemic10,11 and reducing exposure to pollutants10 are necessary to lower COPD risk in developing countries, but do not detail regional differences in risk, outcomes, or treatment options.
The TIOtropium Safety and Performance In Respimat® (TIOSPIR®) study demonstrated similar efficacy and safety profiles between tiotropium Respimat® (2.5 or 5μg) and tiotropium HandiHaler® 18μg, with respect to risk of mortality and risk of exacerbation.12 A recent TIOSPIR® subanalysis demonstrated similar mortality rates in Asian patients versus RoW. Although exacerbation rates were lower in Asia, a higher proportion of patients had severe exacerbations than in RoW.13
The purpose of this post-hoc analysis was to determine whether there are differences in the baseline characteristics, mortality, exacerbations, cardiac adverse event, and serious adverse event (SAE) outcomes of patients from Latin American geographical region versus RoW in the TIOSPIR® trial.
MethodsStudy designTIOSPIR® (NCT01126437) was a large (N=17135), long-term (2–3-year), randomized, double-blind, parallel-group, double-dummy, event-driven trial in patients with COPD comparing the safety and efficacy of once-daily tiotropium (SPIRIVA® [Boehringer Ingelheim, Ingelheim am Rhein, Germany]) via Respimat® 5μg (2 inhalations of 2.5μg), and 2.5μg (2 inhalations of 1.25μg), or via HandiHaler® 18μg; 17116 patients constituted the as-treated population and were included in this analysis. The study design, detailed methods, and primary results were published previously.12,14
Study populationAll participants had a confirmed diagnosis of COPD (forced expiratory volume in 1 second [FEV1] ≤70% predicted and FEV1/forced vital capacity ≤0.70), were aged ≥40 years, and had ≥10 pack-years of smoking history.14 Patients with concomitant cardiac diseases were included in the study, except those with recent (≤6 months) myocardial infarction (MI), severe arrhythmia, or a change in drug therapy within the previous year; or hospitalization for cardiac failure within the previous year.14 All usual background treatments for COPD, except other inhaled anticholinergics, were allowed.14
The geographical region of Latin America enrolled patients in Argentina, Brazil, Colombia, Guatemala, Mexico, Panama, and Peru.
Outcome measuresOutcomes of interest in this analysis were time to all-cause death, time to first moderate-to-severe or severe exacerbation, time to major adverse cardiovascular events (MACE) and fatal MACE, and SAEs for patients in Latin America versus RoW.
AssessmentsAn independent mortality adjudication committee, blinded to treatment assignments attributed the cause of each death.14 Non-fatal stroke and MI (included in MACE) were reported by study investigators and verified for classification accuracy by central reviewers who were blinded to treatment assignment.
Exacerbations were defined as the worsening of ≥2 major respiratory symptoms (dyspnoea, cough, sputum, chest tightness, or wheezing) for ≥3 days and requiring specified treatment changes. Moderate-to-severe exacerbations required a prescription for antibiotics, systemic corticosteroids, or both (with no hospitalization); severe exacerbations required hospitalization. The onset of exacerbation was defined as the onset of the first recorded symptom; the end of exacerbation was decided by the investigator, based on clinical judgment.
A composite endpoint of MACE was included in the analyses, comprising stroke, transient ischaemic attack (TIA), MI, sudden death, cardiac death, sudden cardiac death, or fatal event in the system organ classes (SOCs) for cardiac and vascular disorders. SOCs were defined according to the Medical Dictionary for Regulatory Activities standardizing attributions of AEs globally.
Statistical analysisBased on similar efficacy and safety results among the tiotropium treatment arms in the primary analysis,12 data were pooled for this post-hoc exploratory analysis.
Baseline characteristics discriminating between patients in Latin America and RoW were identified and compared descriptively.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for time to events by region were calculated using a Cox proportional hazards regression model without covariate adjustment, and displayed via Kaplan–Meier plots. A negative binomial regression model was used to estimate the number of exacerbations. HRs for time to death and time to moderate-to-severe exacerbation analyses, by region and baseline characteristics, are shown as forest plots. Additional analyses of time to death and moderate-to-severe exacerbation, severe exacerbations, and MACE were adjusted for covariates including age, post-bronchodilator FEV1% predicted, gender, exacerbation history, smoking history (pack-years), body mass index (BMI), and history of cardiac disorders, MI, coronary artery disease/ischaemic heart disease (CAD/IHD), cardiac arrhythmia, heart failure (class I–IV), and stroke/TIA.
Incidence rates, incidence rate ratios (IRRs), and 95% CIs for causes of death were calculated. SAEs are provided in the Supplementary Material.
For the mortality analysis (vital status), data were included if death occurred up until the study end date, irrespective of treatment discontinuation. For the exacerbation analysis (on-treatment), events were counted from randomization to drug stop date+1 day, and for MACE to drug stop date+30 days.
ResultsStudy populationOf the TIOSPIR® population, 1000 patients from Latin America and 16116 patients from RoW were included (Table 1). Total exposure to tiotropium was 1855 and 32229 patient-years for Latin America and RoW, respectively. A total of 228 (22.8%) patients from Latin America and 3689 (22.9%) patients from RoW discontinued prematurely from trial medication. Reasons for premature discontinuation were similar, and mainly due to AEs, with respiratory disorders (including COPD) being the most common AEs, followed by neoplasms, and infections and infestations.12 Vital status follow-up was complete for 99.1% and 99.8% of patients from Latin America and RoW, respectively.
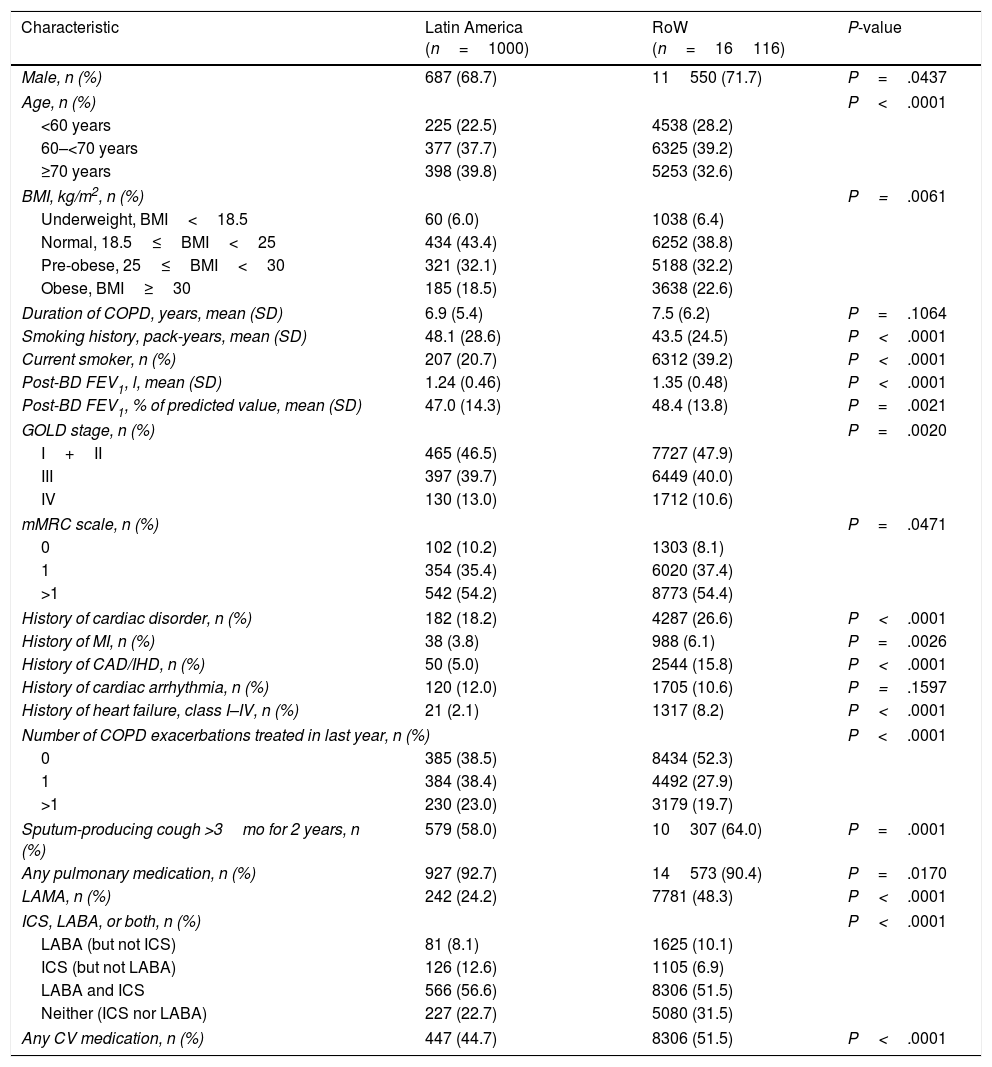
Patient baseline characteristics by region.
| Characteristic | Latin America (n=1000) | RoW (n=16116) | P-value |
|---|---|---|---|
| Male, n (%) | 687 (68.7) | 11550 (71.7) | P=.0437 |
| Age, n (%) | P<.0001 | ||
| <60 years | 225 (22.5) | 4538 (28.2) | |
| 60–<70 years | 377 (37.7) | 6325 (39.2) | |
| ≥70 years | 398 (39.8) | 5253 (32.6) | |
| BMI, kg/m2, n (%) | P=.0061 | ||
| Underweight, BMI<18.5 | 60 (6.0) | 1038 (6.4) | |
| Normal, 18.5≤BMI<25 | 434 (43.4) | 6252 (38.8) | |
| Pre-obese, 25≤BMI<30 | 321 (32.1) | 5188 (32.2) | |
| Obese, BMI≥30 | 185 (18.5) | 3638 (22.6) | |
| Duration of COPD, years, mean (SD) | 6.9 (5.4) | 7.5 (6.2) | P=.1064 |
| Smoking history, pack-years, mean (SD) | 48.1 (28.6) | 43.5 (24.5) | P<.0001 |
| Current smoker, n (%) | 207 (20.7) | 6312 (39.2) | P<.0001 |
| Post-BD FEV1, l, mean (SD) | 1.24 (0.46) | 1.35 (0.48) | P<.0001 |
| Post-BD FEV1, % of predicted value, mean (SD) | 47.0 (14.3) | 48.4 (13.8) | P=.0021 |
| GOLD stage, n (%) | P=.0020 | ||
| I+II | 465 (46.5) | 7727 (47.9) | |
| III | 397 (39.7) | 6449 (40.0) | |
| IV | 130 (13.0) | 1712 (10.6) | |
| mMRC scale, n (%) | P=.0471 | ||
| 0 | 102 (10.2) | 1303 (8.1) | |
| 1 | 354 (35.4) | 6020 (37.4) | |
| >1 | 542 (54.2) | 8773 (54.4) | |
| History of cardiac disorder, n (%) | 182 (18.2) | 4287 (26.6) | P<.0001 |
| History of MI, n (%) | 38 (3.8) | 988 (6.1) | P=.0026 |
| History of CAD/IHD, n (%) | 50 (5.0) | 2544 (15.8) | P<.0001 |
| History of cardiac arrhythmia, n (%) | 120 (12.0) | 1705 (10.6) | P=.1597 |
| History of heart failure, class I–IV, n (%) | 21 (2.1) | 1317 (8.2) | P<.0001 |
| Number of COPD exacerbations treated in last year, n (%) | P<.0001 | ||
| 0 | 385 (38.5) | 8434 (52.3) | |
| 1 | 384 (38.4) | 4492 (27.9) | |
| >1 | 230 (23.0) | 3179 (19.7) | |
| Sputum-producing cough >3mo for 2 years, n (%) | 579 (58.0) | 10307 (64.0) | P=.0001 |
| Any pulmonary medication, n (%) | 927 (92.7) | 14573 (90.4) | P=.0170 |
| LAMA, n (%) | 242 (24.2) | 7781 (48.3) | P<.0001 |
| ICS, LABA, or both, n (%) | P<.0001 | ||
| LABA (but not ICS) | 81 (8.1) | 1625 (10.1) | |
| ICS (but not LABA) | 126 (12.6) | 1105 (6.9) | |
| LABA and ICS | 566 (56.6) | 8306 (51.5) | |
| Neither (ICS nor LABA) | 227 (22.7) | 5080 (31.5) | |
| Any CV medication, n (%) | 447 (44.7) | 8306 (51.5) | P<.0001 |
Patients with missing baseline characteristics are not shown.
Cardiac history was defined as history of MI, CAD/IHD, cardiac arrhythmia, or heart failure. Any CV medication includes β-blockers, calcium channel blockers, cardiac glycosides (digoxin), angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, nitrates, anti-arrhythmics class I or III (sodium or potassium channel blockers), adenosine, acetylsalicylic acid, anticoagulants (vitamin K antagonists, direct thrombin inhibitors, and factor Xa inhibitors), and antiplatelets.
BD, bronchodilator; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; IHD, ischaemic heart disease; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MI, myocardial infarction; mMRC, Modified Medical Research Council; RoW, rest of the world; SD, standard deviation.
At baseline, patients enrolled in Latin America were comparatively older (higher proportion of patients aged ≥70 years), and had higher mean pack-years of smoking history than those in RoW, although a lower percentage were current smokers (Table 1). A higher proportion of patients in Latin America had COPD exacerbation in the year prior to the trial than those in RoW. Latin American patients were more likely to have very severe COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] Stage IV) versus RoW (Table 1). Fewer Latin American patients had a history of cardiac disorders, or were receiving cardiovascular medication at baseline versus RoW (Table 1). The proportions of patients receiving long-acting muscarinic antagonist (LAMA) or long-acting β2-agonist (LABA) at baseline were lower in Latin America than in RoW; however, they were more likely to receive inhaled corticosteroids (ICS) (with or without LABA) (Table 1).
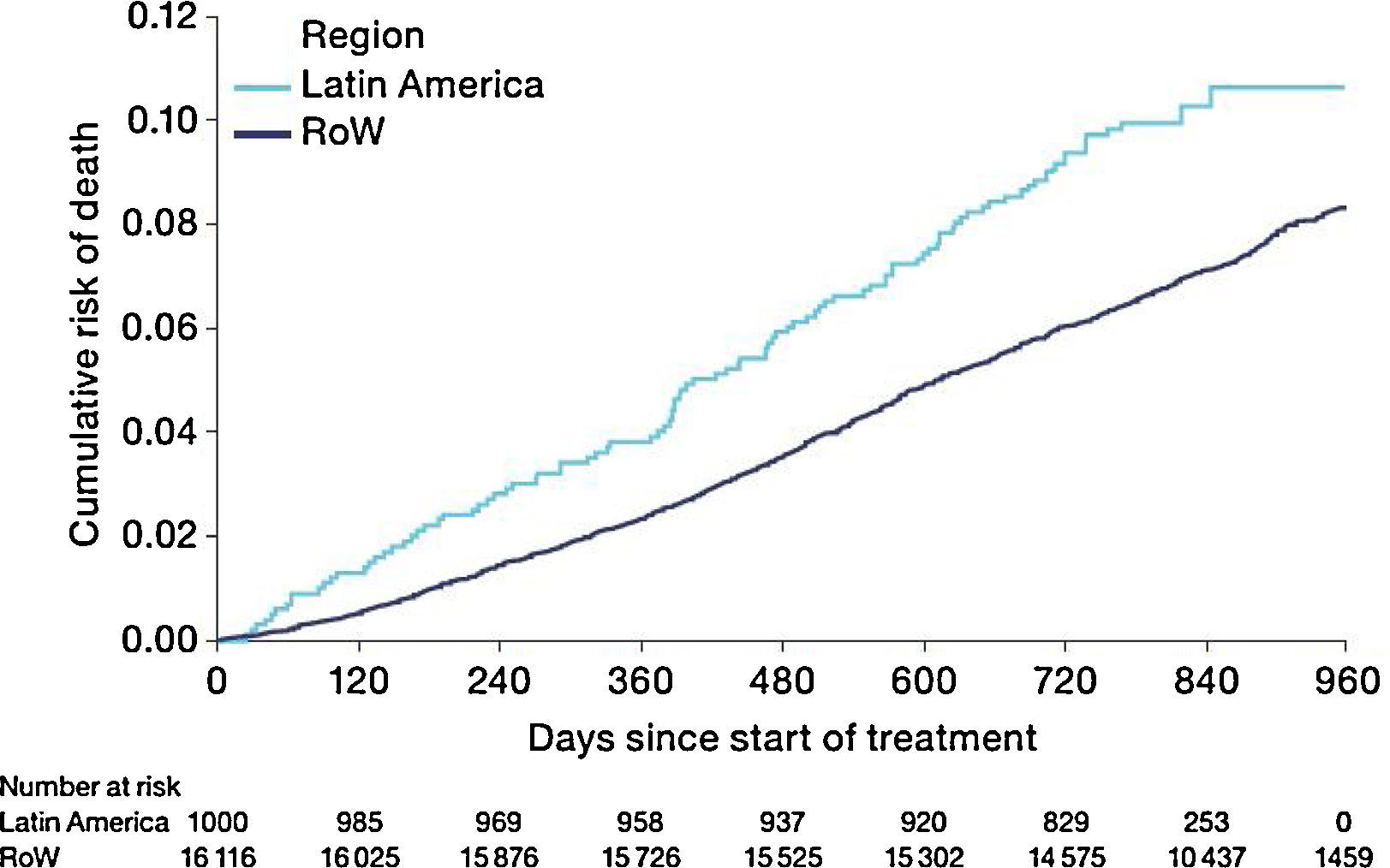
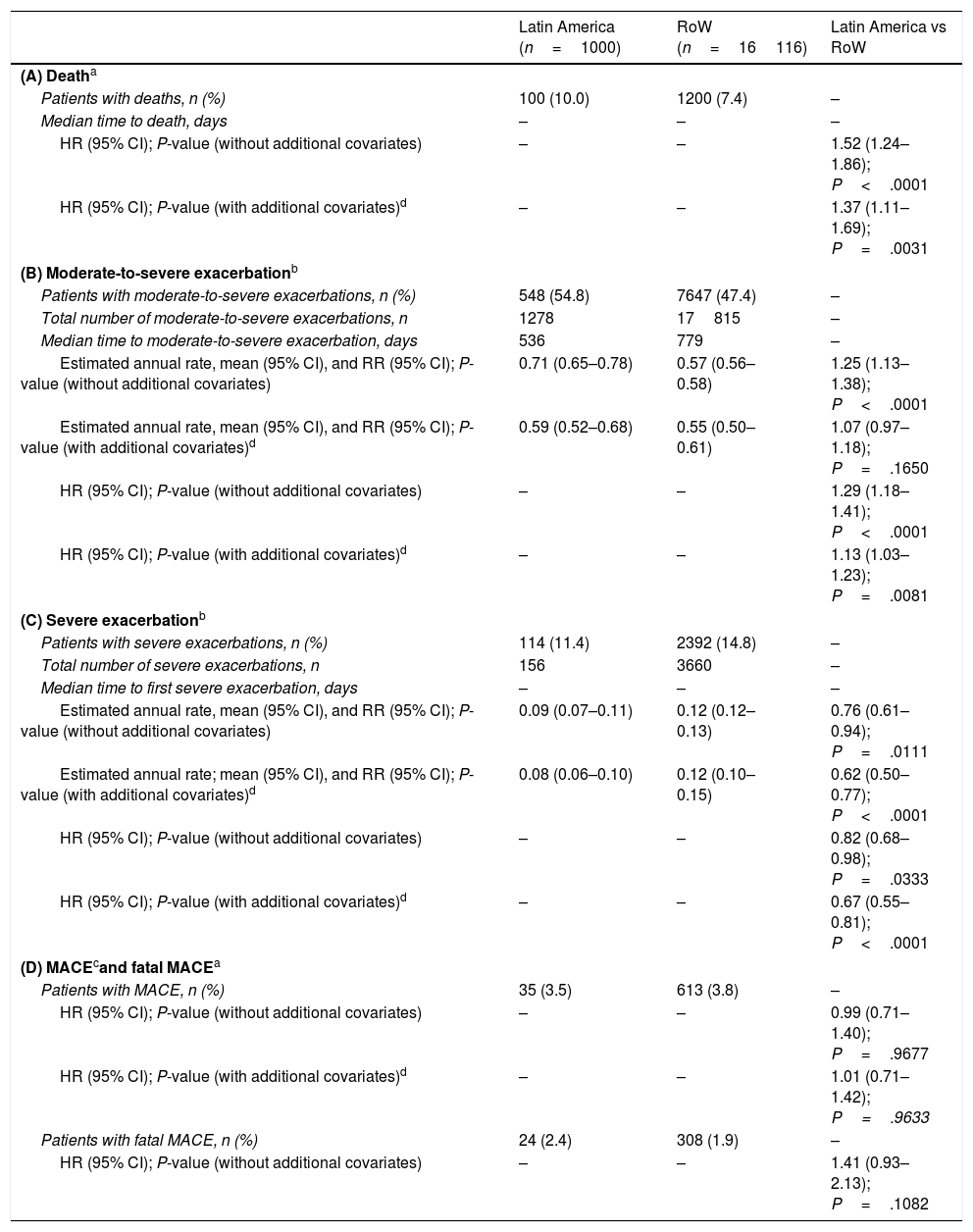
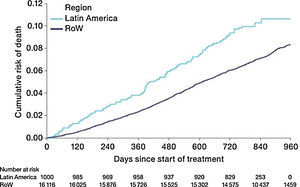
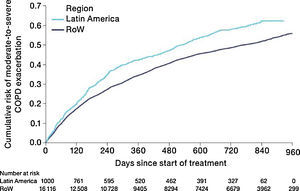
MortalityDuring the study, a greater proportion of patients died in the Latin American population (n=100 [10.0%]) versus RoW (n=1200 [7.4%]) (Table 2A). Analysis of time to death confirmed that patients in Latin America had a higher risk of death than those in RoW (HR [95% CI]: 1.52 [1.24–1.86]) (Table 2A; Fig. 1). This was also true when the analysis of time to death was adjusted for age, gender, FEV1% predicted, exacerbation history, smoking history (pack-years), BMI, history of cardiac disorders, MI, CAD/IHD, cardiac arrhythmia, heart failure (class I–IV), and stroke/TIA (HR [95% CI]: 1.37 [1.11–1.69]) (Table 2A).
| Latin America (n=1000) | RoW (n=16116) | Latin America vs RoW | |
|---|---|---|---|
| (A) Deatha | |||
| Patients with deaths, n (%) | 100 (10.0) | 1200 (7.4) | – |
| Median time to death, days | – | – | – |
| HR (95% CI); P-value (without additional covariates) | – | – | 1.52 (1.24–1.86); P<.0001 |
| HR (95% CI); P-value (with additional covariates)d | – | – | 1.37 (1.11–1.69); P=.0031 |
| (B) Moderate-to-severe exacerbationb | |||
| Patients with moderate-to-severe exacerbations, n (%) | 548 (54.8) | 7647 (47.4) | – |
| Total number of moderate-to-severe exacerbations, n | 1278 | 17815 | – |
| Median time to moderate-to-severe exacerbation, days | 536 | 779 | – |
| Estimated annual rate, mean (95% CI), and RR (95% CI); P-value (without additional covariates) | 0.71 (0.65–0.78) | 0.57 (0.56–0.58) | 1.25 (1.13–1.38); P<.0001 |
| Estimated annual rate, mean (95% CI), and RR (95% CI); P-value (with additional covariates)d | 0.59 (0.52–0.68) | 0.55 (0.50–0.61) | 1.07 (0.97–1.18); P=.1650 |
| HR (95% CI); P-value (without additional covariates) | – | – | 1.29 (1.18–1.41); P<.0001 |
| HR (95% CI); P-value (with additional covariates)d | – | – | 1.13 (1.03–1.23); P=.0081 |
| (C) Severe exacerbationb | |||
| Patients with severe exacerbations, n (%) | 114 (11.4) | 2392 (14.8) | – |
| Total number of severe exacerbations, n | 156 | 3660 | – |
| Median time to first severe exacerbation, days | – | – | – |
| Estimated annual rate, mean (95% CI), and RR (95% CI); P-value (without additional covariates) | 0.09 (0.07–0.11) | 0.12 (0.12–0.13) | 0.76 (0.61–0.94); P=.0111 |
| Estimated annual rate; mean (95% CI), and RR (95% CI); P-value (with additional covariates)d | 0.08 (0.06–0.10) | 0.12 (0.10–0.15) | 0.62 (0.50–0.77); P<.0001 |
| HR (95% CI); P-value (without additional covariates) | – | – | 0.82 (0.68–0.98); P=.0333 |
| HR (95% CI); P-value (with additional covariates)d | – | – | 0.67 (0.55–0.81); P<.0001 |
| (D) MACEcand fatal MACEa | |||
| Patients with MACE, n (%) | 35 (3.5) | 613 (3.8) | – |
| HR (95% CI); P-value (without additional covariates) | – | – | 0.99 (0.71–1.40); P=.9677 |
| HR (95% CI); P-value (with additional covariates)d | – | – | 1.01 (0.71–1.42); P=.9633 |
| Patients with fatal MACE, n (%) | 24 (2.4) | 308 (1.9) | – |
| HR (95% CI); P-value (without additional covariates) | – | – | 1.41 (0.93–2.13); P=.1082 |
Adjusted for age, FEV1% predicted, gender, exacerbation history, smoking history (pack-years), body mass index, and history of cardiac disorders, myocardial infarction, coronary artery disease/ischaemic heart disease, cardiac arrhythmia, heart failure (class I–IV), and stroke/transient ischemic attack. Patients with missing covariates (n=6 for Latin America and n=45 for RoW) are excluded.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; HR, hazard ratio; MACE, major adverse cardiovascular events; RoW, rest of the world; RR, rate ratio.
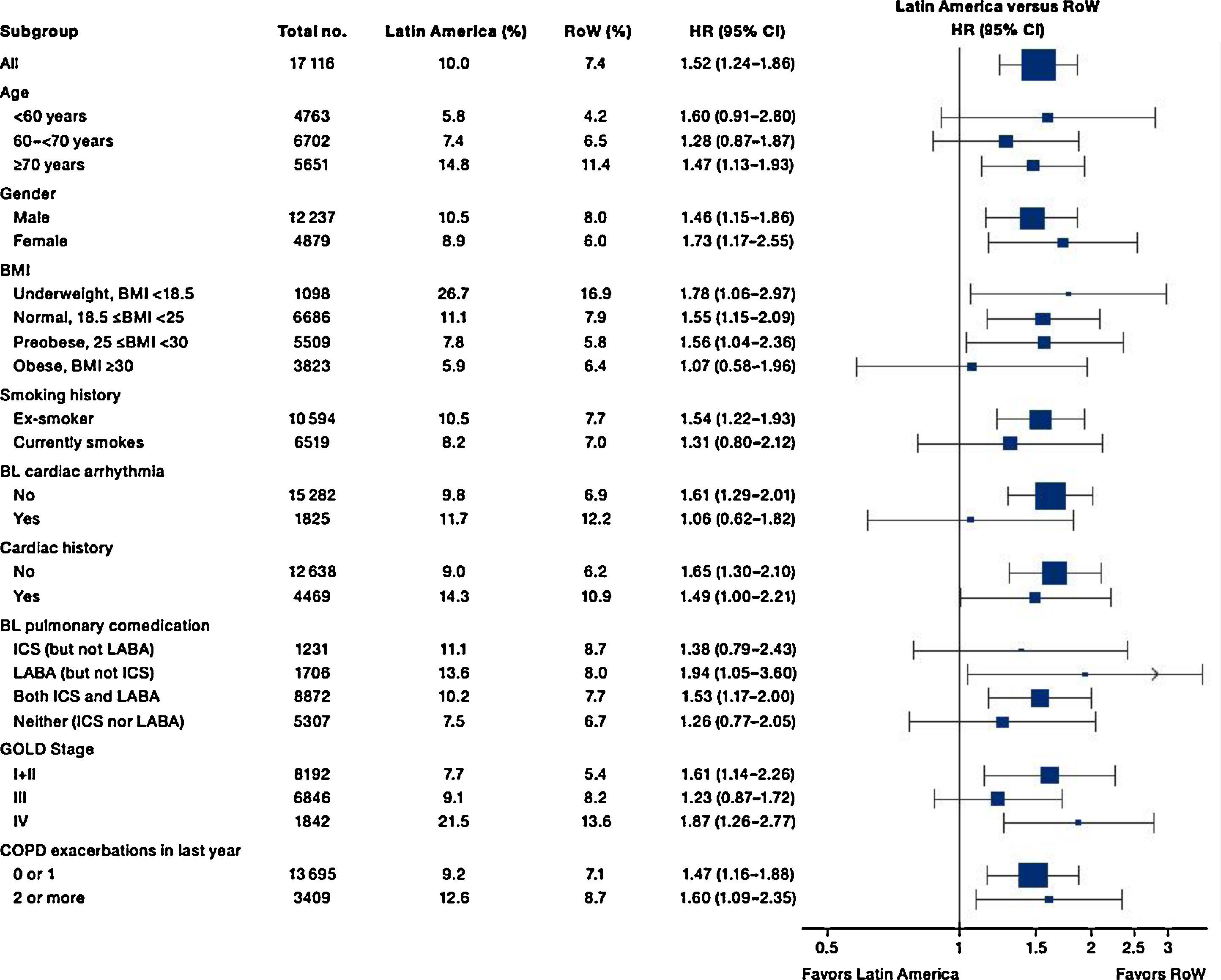
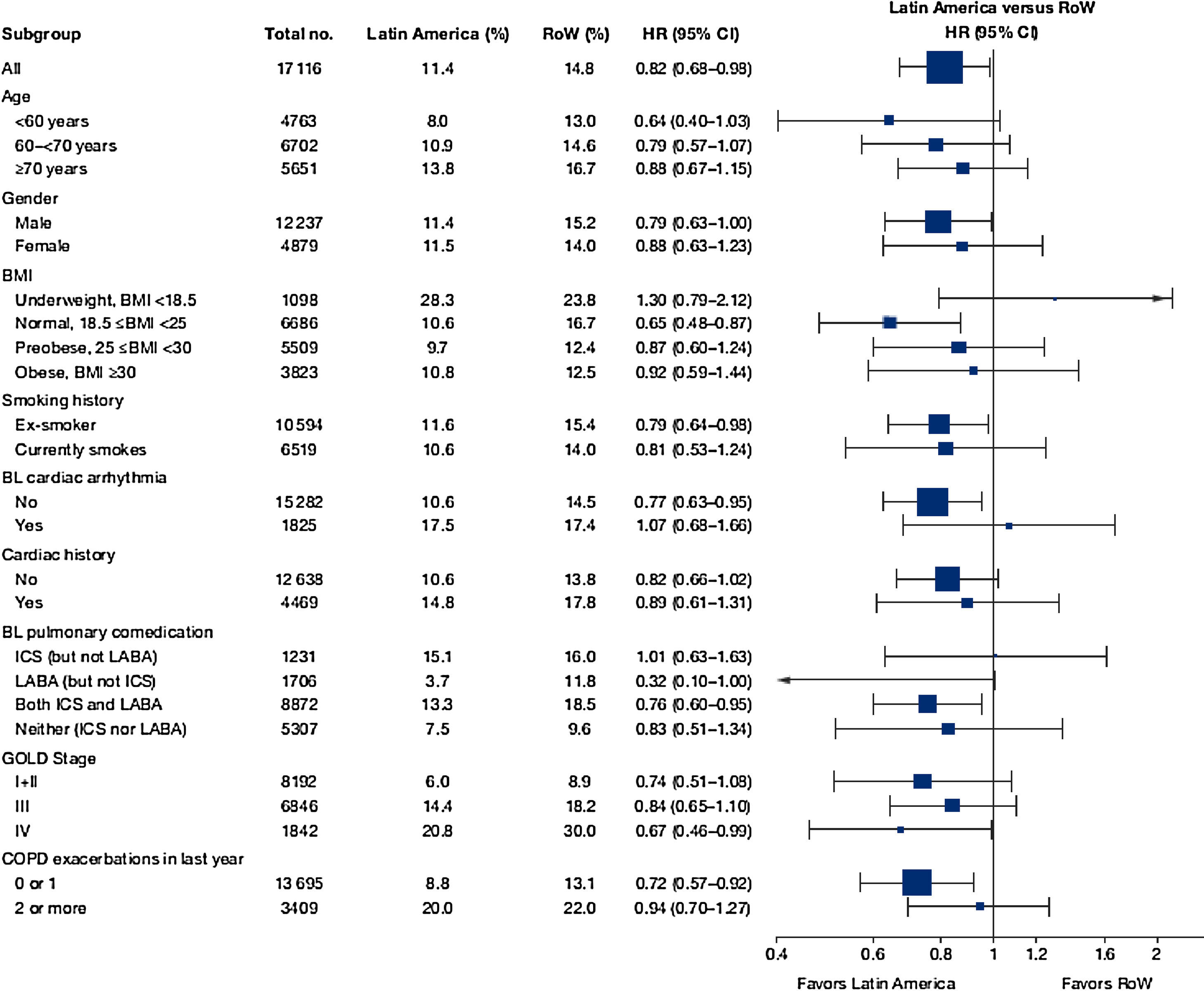
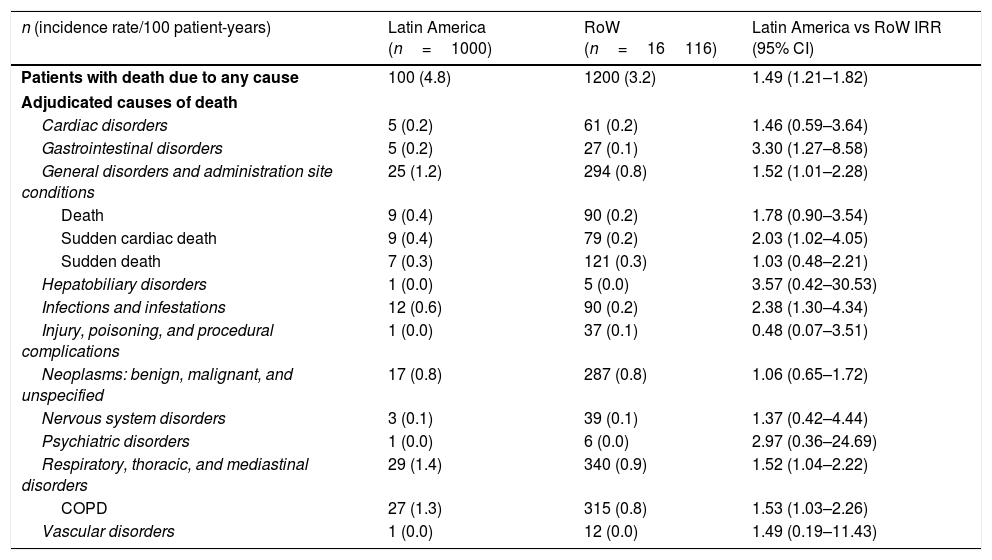
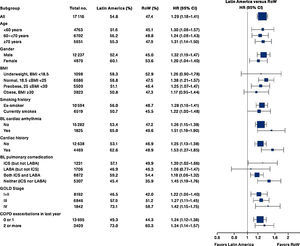
While the overall risk of death was increased in patients from Latin America versus RoW, regression analysis according to baseline characteristics showed that the risk of death was similar for Latin America and RoW in patients with BMI ≥30kg/m2 (HR [95% CI]: 1.07 [0.58–1.96]), and patients with cardiac arrhythmia (HR [95% CI]: 1.06 [0.62–1.82]) (Fig. 2). The largest HRs were observed in patients with GOLD Stage IV (HR [95% CI]: 1.87 [1.26–2.77]) and in those who received LABA (but no ICS) (HR [95% CI]: 1.94 [1.05–3.60]) (Fig. 2), however the LABA (no ICS) result is based on small patient number. Causes of death were mainly similar between the regions, except for respiratory, thoracic, and mediastinal disorders, including COPD, gastrointestinal disorders, general disorders including death due to unknown reason (sudden cardiac death and death), and infections and infestations, where the incidence rate was higher in the Latin American region versus RoW. General disorders, neoplasms, and respiratory disorders, including COPD, contributed the majority of deaths (Table 3).
Time to death by region and according to baseline characteristics (vital status analysis).
Data were included if the death occurred up until the end of the study.
BL, baseline; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; RoW, rest of the world.
Adjudicated causes of death by region (vital status analysis).
| n (incidence rate/100 patient-years) | Latin America (n=1000) | RoW (n=16116) | Latin America vs RoW IRR (95% CI) |
|---|---|---|---|
| Patients with death due to any cause | 100 (4.8) | 1200 (3.2) | 1.49 (1.21–1.82) |
| Adjudicated causes of death | |||
| Cardiac disorders | 5 (0.2) | 61 (0.2) | 1.46 (0.59–3.64) |
| Gastrointestinal disorders | 5 (0.2) | 27 (0.1) | 3.30 (1.27–8.58) |
| General disorders and administration site conditions | 25 (1.2) | 294 (0.8) | 1.52 (1.01–2.28) |
| Death | 9 (0.4) | 90 (0.2) | 1.78 (0.90–3.54) |
| Sudden cardiac death | 9 (0.4) | 79 (0.2) | 2.03 (1.02–4.05) |
| Sudden death | 7 (0.3) | 121 (0.3) | 1.03 (0.48–2.21) |
| Hepatobiliary disorders | 1 (0.0) | 5 (0.0) | 3.57 (0.42–30.53) |
| Infections and infestations | 12 (0.6) | 90 (0.2) | 2.38 (1.30–4.34) |
| Injury, poisoning, and procedural complications | 1 (0.0) | 37 (0.1) | 0.48 (0.07–3.51) |
| Neoplasms: benign, malignant, and unspecified | 17 (0.8) | 287 (0.8) | 1.06 (0.65–1.72) |
| Nervous system disorders | 3 (0.1) | 39 (0.1) | 1.37 (0.42–4.44) |
| Psychiatric disorders | 1 (0.0) | 6 (0.0) | 2.97 (0.36–24.69) |
| Respiratory, thoracic, and mediastinal disorders | 29 (1.4) | 340 (0.9) | 1.52 (1.04–2.22) |
| COPD | 27 (1.3) | 315 (0.8) | 1.53 (1.03–2.26) |
| Vascular disorders | 1 (0.0) | 12 (0.0) | 1.49 (0.19–11.43) |
CI, confidence interval; COPD, chronic obstructive pulmonary disease; IRR, incidence rate ratio; RoW, rest of the world.
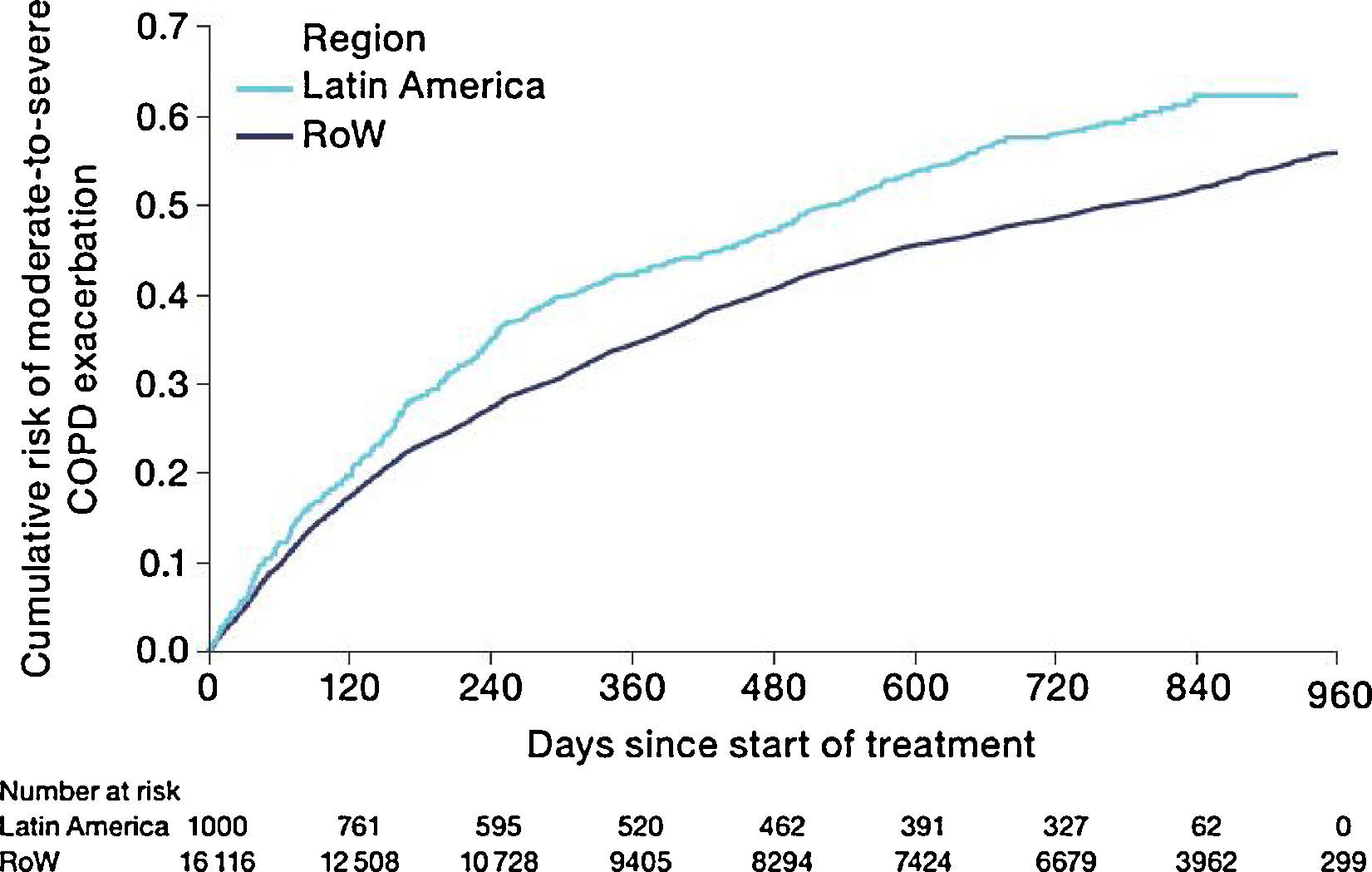
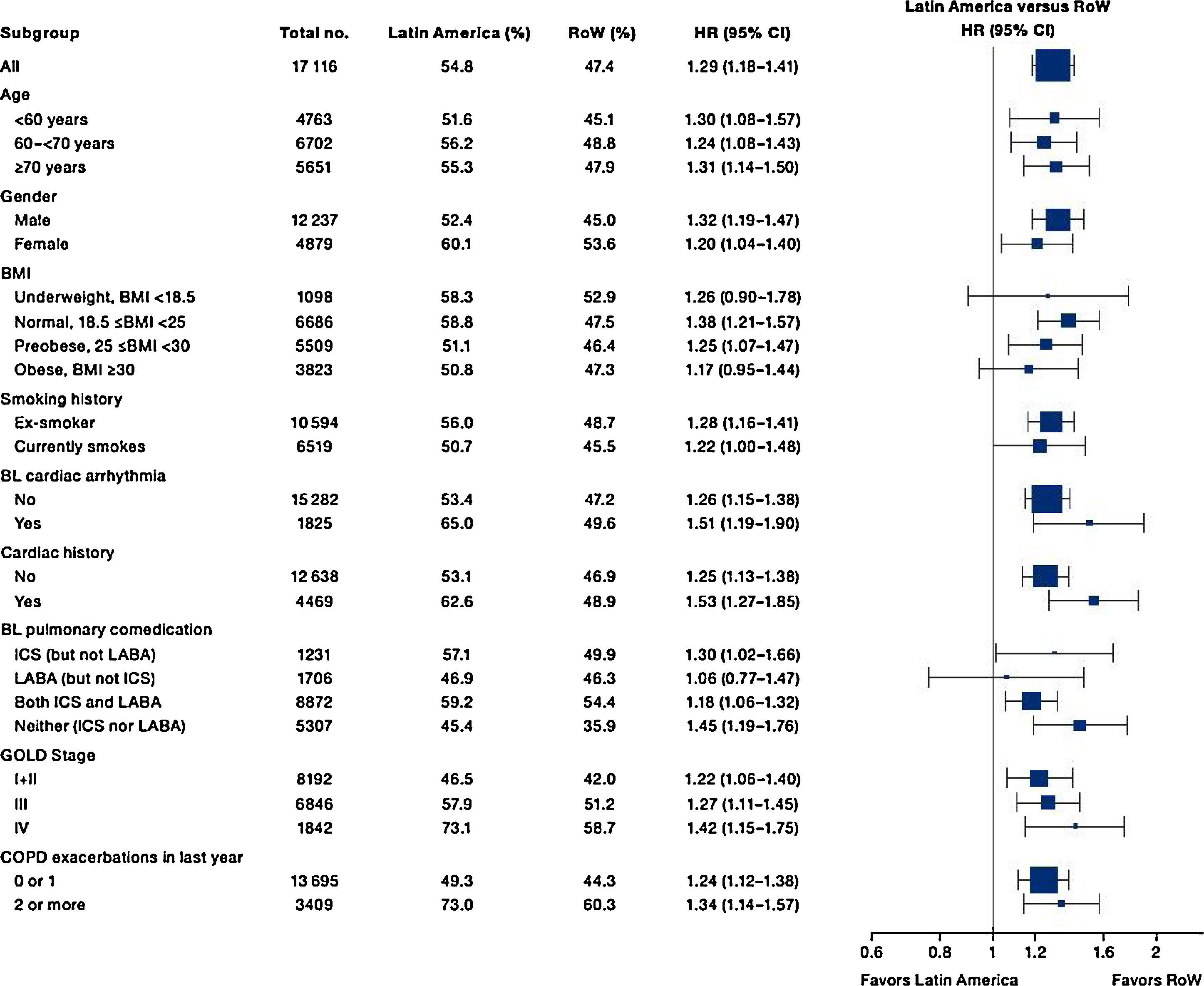
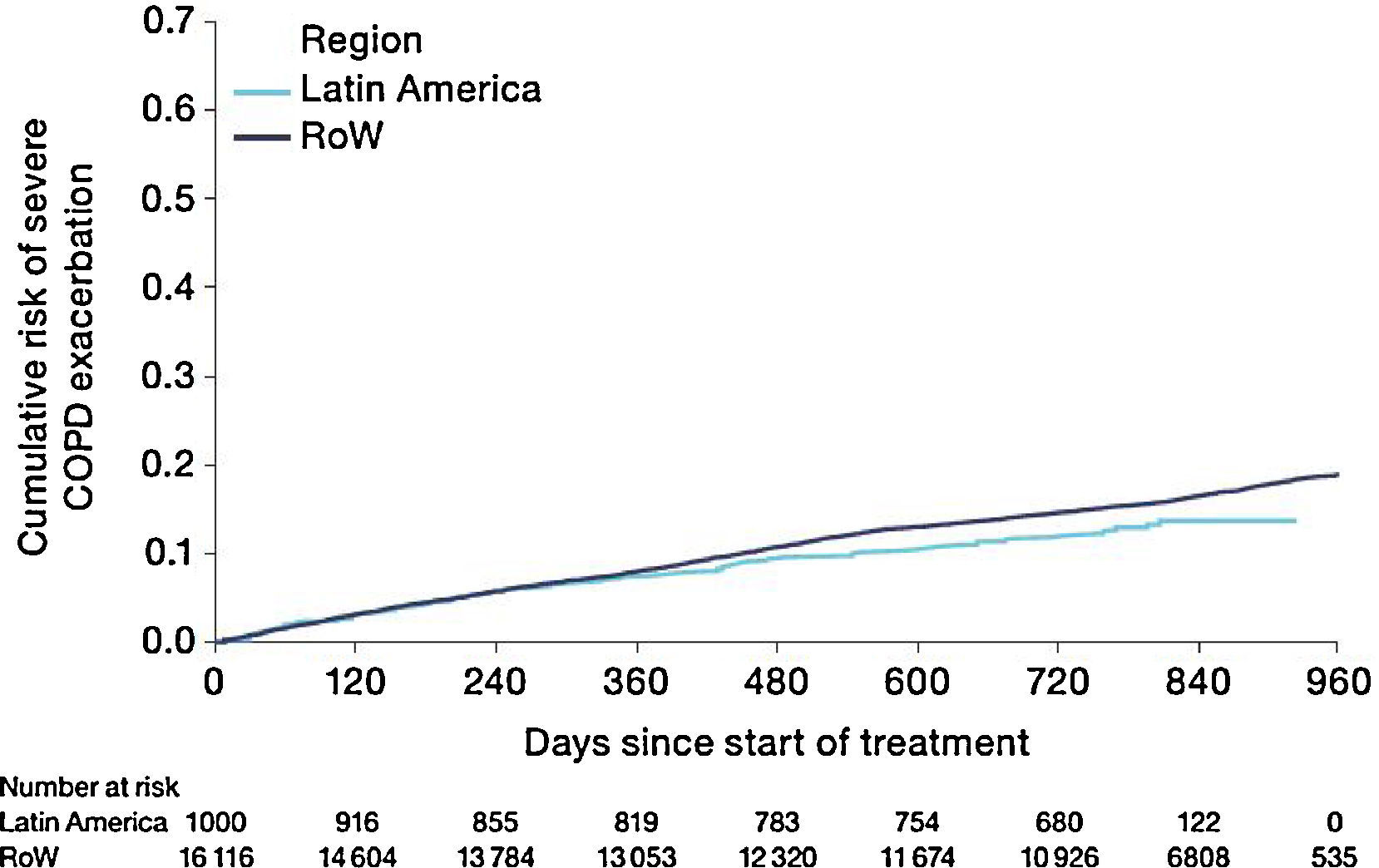
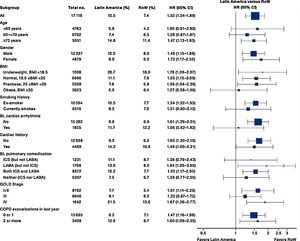
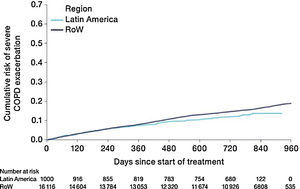
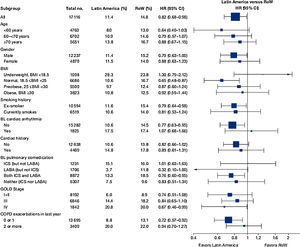
Similar to the mortality analysis, the risk of moderate-to-severe exacerbation (time to first moderate-to-severe exacerbation) was higher in patients in Latin America versus RoW (HR [95% CI]: 1.29 [1.18–1.41]) (Table 2B; Fig. 3). Additional analysis adjusting for relevant covariates confirmed a higher risk of moderate-to-severe exacerbations in Latin American patients versus RoW (HR [95% CI]: 1.13 [1.03–1.23]) (Table 2B). Accordingly, the annual rate of moderate-to-severe exacerbations was higher in Latin America (RR [95% CI]: 1.25 [1.13–1.38]) even when adjusted for covariates (RR [95% CI]: 1.07 [0.97–1.18]) (Table 2B). Time to first moderate-to-severe exacerbation according to baseline characteristics confirmed the increased risk in Latin America versus RoW, except for patients receiving LABA (but no ICS) at baseline (HR [95% CI]: 1.06 [0.77–1.47]), where the risk was similar between both regions (Fig. 4). In contrast, patients in Latin America had a lower risk of severe COPD exacerbation (longer time to first severe exacerbation leading to hospitalization) (HR [95% CI]: 0.82 [0.68–0.98]) (Table 2C; Fig. 5), including when adjusted for covariates (HR [95% CI]: 0.67 [0.55–0.81]) (Table 2C). Severe exacerbations also occurred less frequently versus RoW (RR [95% CI]: 0.76 [0.61–0.94]) (Table 2C), and similar results were obtained when adjusted for covariates (RR [95% CI]: 0.62 [0.50–0.77]) (Table 2C). Time to first severe exacerbation according to baseline characteristics adjusted for covariates supported the lower risk of severe exacerbation in Latin American patients versus RoW, except for patients receiving ICS (but no LABA) at baseline (HR [95% CI]: 1.01 [0.63–1.63]) and patients with cardiac arrhythmia (HR [95%CI]: 1.07 [0.68–1.66]). However, an increased risk of severe exacerbations was observed in underweight (BMI<18.5) patients in Latin America versus RoW (HR [95%CI]: 1.30 [0.79–2.12]) (Fig. 6).
Time to first moderate-to-severe COPD exacerbation by region and according to baseline characteristics (on-treatment analysis).
Events were counted from randomization to drug stop date+1 day.
BL, baseline; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; RoW, rest of the world.
Time to first severe COPD exacerbation by region adjusted for relevant covariatesa (on-treatment analysis).
Events were counted from randomization to drug stop date+1 day.
aAdjusted for age, FEV1% predicted, gender, exacerbation history, smoking history (pack-years), BMI, history of cardiac disorders, myocardial infarction, coronary artery disease/ischaemic heart disease, cardiac arrhythmia, heart failure (class I–IV), and stroke/transient ischemic attack. Patients with missing covariates (n=6 for Latin America and n=45 for RoW) are excluded.
BL, baseline; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; RoW, rest of the world.
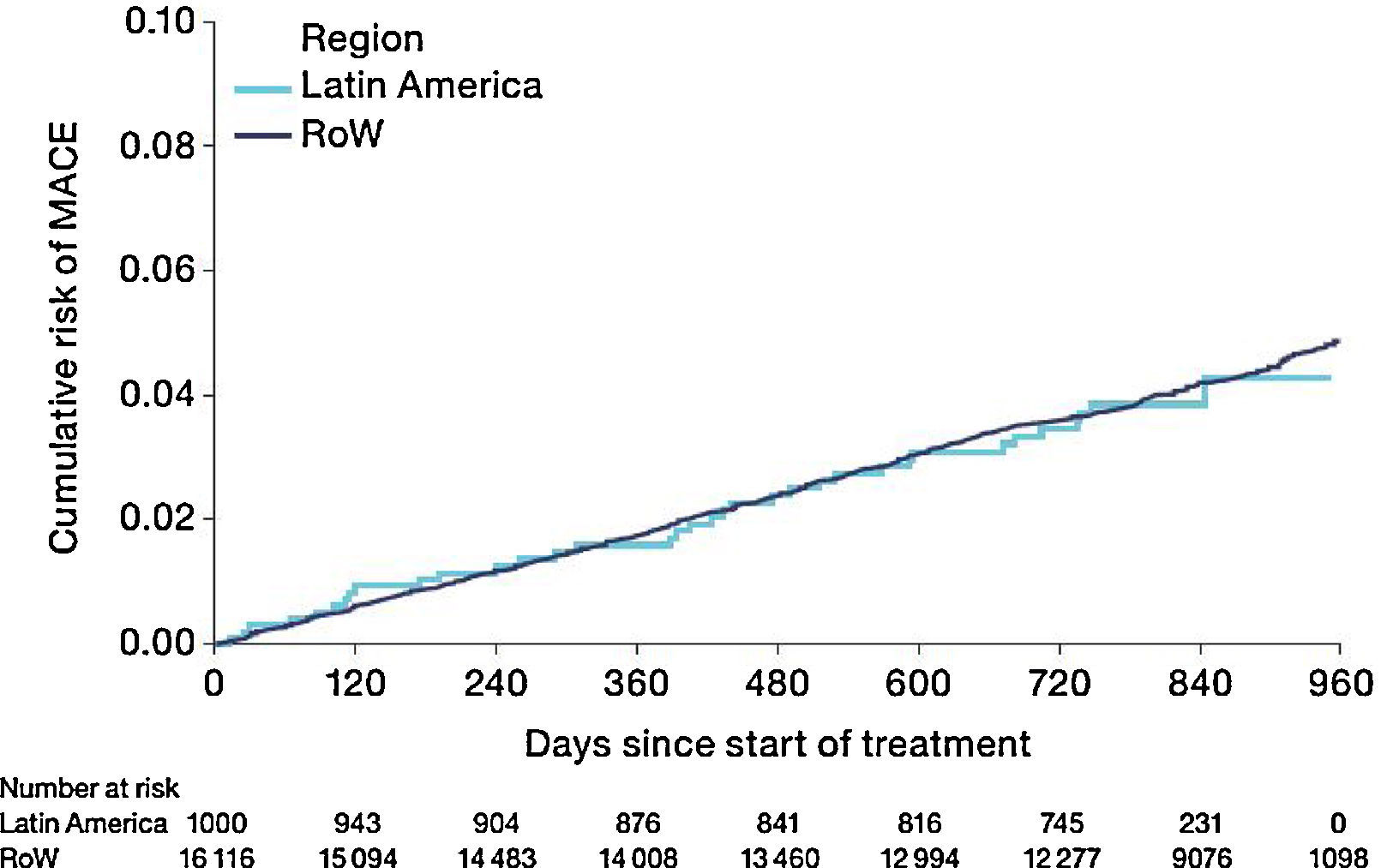
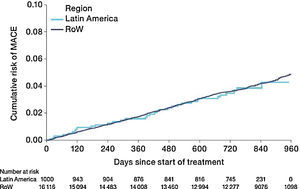
The risk of MACE (HR [95% CI]: 0.99 [0.71–1.40]) was similar between Latin America and RoW (Fig. 7) even when adjusted for covariates (HR [95% CI]: 1.01 [0.71–1.42]) (Table 2D). However, the risk of fatal MACE (HR [95% CI]: 1.41 [0.93–2.13]) (Table 2D) was numerically higher in Latin America versus RoW, although only a small proportion of patients had fatal MACE (2.4% in Latin America vs 1.9% in RoW; Table 2D).
Time to first MACE by region (on-treatment analysis).
Events were counted from randomization to drug stop date+30 days.
MACE includes stroke, transient ischaemic attack, MI, sudden death, cardiac death, sudden cardiac death, or fatal event in SOCs for cardiac and vascular disorders.
SOCs were defined according to MedDRA and PT.
MACE, major adverse cardiovascular event; MedDRA, Medical Dictionary for Regulatory Activities; MI, myocardial infarction; PT, preferred term; RoW, rest of the world; SOC, system organ class.
Incidence rates of SAEs were lower in Latin America than in the RoW, including cardiac disorders, cancer, nervous system disorders, and overall respiratory disorders including COPD (Table S1).
Discussion and conclusionsLatin America is undergoing demographic changes characterized by increased ageing of the population, and the higher proportion of patients aged ≥60 years in Latin American countries is a reflection of this statistic.7,15 As expected, Latin American patients also had higher pack-years of smoking history versus RoW, although current smokers were less frequent in Latin America, indicating that more patients had stopped smoking prior to study start.
Compared with RoW, more patients in Latin America had very severe COPD (GOLD Stage IV) at baseline, possibly indicating that these patients remained undiagnosed during the early stages of COPD, and reflecting suboptimal disease management in this region.15,16 The PLATINO study, identified underdiagnosis as an issue across Latin America,17 while more than half of patients included in the PUMA study were not using bronchodilators.18 Our findings support this earlier research, with few patients using bronchodilators at baseline, suggesting a significant gap between guideline recommendations and prescribing patterns in this region.18 A scarcity of respiratory specialists and training programmes in many Latin American countries7 is likely to increase the risk of poor clinical outcomes.10 These factors could also have contributed to the observed higher proportion of patients with exacerbations in the year prior to our study. Thorough assessment, accurate diagnosis, and prompt initiation of management strategies (such as smoking cessation and maintenance respiratory therapy) in COPD are known to be essential for mitigating future risks.7,10,15 Evidence-based literature recommends smoking cessation at early stages of COPD.19
Latin American patients had increased risk of death compared with RoW, even when adjusted for age, gender, FEV1% predicted, exacerbation history, smoking history (pack-years), BMI, and history of cardiac disorders, MI, CAD/IHD, cardiac arrhythmia, heart failure (class I–IV), and stroke/TIA. These factors are proposed drivers of mortality and morbidity in COPD10,20,21 and are likely to be relevant regardless of geographical location. Findings from the PLATINO study also support an association between COPD and increased mortality when adjusted by age, smoking status, pack-years smoking, quality of life, BMI, and comorbidities.4 Comparable with previous findings,4 the incidence rate of death due to respiratory, thoracic, and mediastinal disorders, including COPD, was higher in Latin America versus RoW in our study.
Latin American patients also had a higher risk of moderate-to-severe exacerbation than patients in RoW. The risk was consistently higher in most subgroups, except for patients receiving LABA (but not ICS) at baseline. Nevertheless, although Latin American patients presented with more severe disease at baseline, and higher mortality or moderate-to-severe exacerbation risks, our analysis demonstrated that they were less likely to have severe exacerbation than RoW. This was true even when the analysis was adjusted for covariates, except for patients receiving ICS at baseline and patients with cardiac arrhythmia; underweight patients had an increased risk in Latin America versus RoW. Fewer severe exacerbations were recorded as a result of the scarcity of admission beds22 in Latin American hospitals, or related healthcare challenges such as inadequate facilities for critical care units.7 Our findings were contrary to those of several Latin American epidemiology studies, which found that hospitalization due to exacerbation was commonplace, reflecting undertreatment.15,16,18 In the PUMA study, frequent overtreatment with corticosteroids was observed in patients without an exacerbation in the past year, while a lower hospitalization rate was not deemed to support the extensive use of corticosteroids.16 Our results showed that corticosteroid overuse was prevalent in Latin America, as more patients were likely to receive ICS at baseline versus RoW.
Unlike the regional differences we identified for Latin American patients, the TIOSPIR® subanalysis of an Asian cohort observed similar mortality rates in Asian patients versus RoW patients.13 Further, Asian patients reported fewer exacerbations than those in RoW, whereas the proportion with a severe exacerbation was higher.13 This may be attributed to differences in the healthcare system in different regions; for example, in Asia, patients are frequently hospitalized for an exacerbation due to non-availability of corticosteroids or antibiotics in a primary healthcare unit.13 The observed discrepancies between the results for the Asian and the Latin American cohort illustrate how racial and/or geographical differences can impact hospitalization rates and treatment outcomes in COPD trials.
Patients enrolled in TIOSPIR® in Latin America were less likely to have a history of cardiac disorders or to be receiving cardiovascular medications at baseline compared with RoW. Assuming that their initial cardiovascular comorbidity was indeed lower, it is therefore rational that Latin American patients were less likely to experience a cardiac disorder SAE than those in RoW during the trial. The composite outcome of MACE was actually similar for both regions, though the risk of fatal MACE was numerically increased in Latin American patients. Further research is needed to clarify relative cardiac risks in this cohort, and the predisposing factors.
It was beyond the scope of our analysis to assess differences in baseline characteristics and outcomes between individual countries in the Latin American region, as the sample sizes may be too small to enable meaningful assessment. Environmental factors not assessed here also influence COPD-related risks, such as access to healthcare and alternative disease management strategies,7,23–25 the extent of solid fuel use,10 or living at high altitude. Hypoxia can have unwanted extra pulmonary systemic effects in patients with COPD, and might be associated with high altitude and mortality from COPD.26 Temperature inversions can also result in a buildup of air pollution that is detrimental to those with respiratory conditions.27 In addition, patients with COPD in tropical countries (such as Brazil, Columbia, and Peru) may be particularly prone to exacerbations, as infections are more prevalent throughout the year, and not just in the winter months (owing to the high humidity and lack of seasonal variation in temperature).28
The large population size and 2- to 3-year duration of the TIOSPIR® trial, along with the wide range of nationalities included (50 countries across different regions of the world14), constitute strengths of this analysis. Patients in TIOSPIR® constituted a convenience sample from 7 Latin American countries (rather than a sample representative of the whole population) and should therefore be interpreted with caution if generalized to Latin America overall. However, the patient characteristics are representative of the patients seen in specialist practice globally, and the continuation of regular maintenance respiratory medication and inclusion of patients with cardiac disease ensured that the population reflects real-world patients.12,14 Thus, we feel that the results are likely to be valid in a clinical setting. However, as this was a post-hoc rather than a predefined subgroup analysis, it is not possible to confirm the reasons why outcome risks differed between Latin America and RoW.
Latin American patients with COPD in TIOSPIR® had a higher risk of death and COPD exacerbations versus RoW patients, but were less likely to be hospitalized for an exacerbation. The prevalence of tobacco smoking, exposure to environmental contaminants, or healthcare challenges might impact treatment outcomes and lead to different results for patients enrolled from various regions in international trials of COPD. This should be considered when designing trials and evaluating the global clinical relevance of the results. Further studies should explore factors influencing the increased risk of mortality in Latin America compared with RoW.
FundingThe TIOSPIR® trial and this post-hoc study were funded by Boehringer Ingelheim.
AuthorshipAll authors confirm involvement in the conception, hypotheses delineation, and design of the study; acquisition, analysis, or interpretation of the data; writing or substantial involvement in the article's revision prior to submission.
Conflict of interestAA reports receiving consulting fees, lecture fees, or travel support from AstraZeneca, Boehringer Ingelheim, Forest Laboratories, GlaxoSmithKline, and Novartis. PMAC has received research grants from GlaxoSmithKline and Takeda; personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Takeda; and non-financial support from Boehringer Ingelheim. AM, NM, and MH are employees of Boehringer Ingelheim. JRJ reports receiving consulting fees, lecture fees, or travel support from AstraZeneca, Boehringer Ingelheim, Novartis, Grifols, CSL, Zambon, EMS, and Chiesi in the past 12 months. EP reports receiving consulting fees, lecture fees, or travel support from Ache, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Chiesi in the past 12 months; EP is currently a Global Medical Expert for GlaxoSmithKline. HG is an advisory member of Boehringer Ingelheim, Novartis, GSK, AstraZeneca, and Mundipharma; and has received lecture fees from Novartis, and travel support from MSD and AstraZeneca. ARV reports travel support from AstraZeneca and Novartis in the past 12 months. ERG reports receiving travel support, consulting fees and lecture fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim and Mundipharma.
Editorial and writing support was provided by Jennifer Fuchs and Deepti Sharda at PAREXEL, funded by Boehringer Ingelheim.