The genus Cryptococcus includes different species of encapsulated yeast fungi, of which only C. neoformans is considered a human pathogen. Its polysaccharide capsule confers virulence by protecting it from phagocytosis and complement activity. Four serotypes within this species have been described — A, B, C, and D — depending on the components of the capsule. Serotypes A and D are identified as C. neoformans var. neoformans, and antigens B and C as C. neoformans var. gattii. The 2 varieties differ both in their pathogenesis and their geographical distribution. C. neoformans var. neoformans is distributed worldwide and is associated with infections in immunocompromised patients, while C. neoformans var. gattii has been associated with infections in immunocompetent patients, and its distribution is more restricted to tropical and subtropical countries.1
C. neoformans var. neoformans can affect any individual, although it is more common in patients with a predisposing factor (HIV infection, use of immunosuppressive drugs, connective tissue disease, cirrhosis, etc.).2
Despite the fact that the pigeon feces are the most important source of infection, these animals do not suffer from the disease. Humans acquire Cryptococcus infection by the respiratory route, and transmission from person to person has not been proven. Although the infection tends to enter via the airways, pulmonary involvement is rare, while the most common presentation is neurological. Pulmonary lesions caused by Cryptococcus vary, and include nodules, masses, interstitial infiltrates, alveolar consolidation, and lymphadenopathy.3,4 Pleural effusion, either isolated or associated with pulmonary disease is a rare manifestation.2–6
We describe a case of pleuritis caused by C. neoformans in an immunocompetent patient.
Our patient was a 78-year-old man with a history of chronic kidney disease stage 3a, permanent atrial fibrillation, congestive heart failure with preserved ejection fraction, alcoholic liver disease, and chronic obstructive pulmonary disease/sleep apnea hypopnea syndrome overlap (COPD + SAHS) receiving treatment with CPAP. He attended our clinic due to a 4 or 5-day-history of sudden onset right pleuritic pain, accompanied by increased dyspnea, cough with expectoration of mucus, and low-grade fever in the afternoon. His general status on physical examination was good, with blood pressure 139/68 mmHg, heart rate 83 bpm, axillary temperature 37.5 °C, SatO2 95 % baseline. Mobile right axillary lymphadenopathies were detected, with no palpable lymphadenopathies in other territories. Arrhythmias were heard on cardiac auscultation, and pulmonary auscultation revealed reduced breath sounds in the right lung base with bilateral rhonchi; no other significant findings were detected on physical examination.
Clinical laboratory tests were significant for mild anemia and raised inflammatory markers. Chest radiography revealed right pleural effusion.
A diagnostic thoracentesis was performed, and the drained fluid showed biochemical characteristics of exudate: pH 7.45, glucose 121 mg/dl, protein 4.1 g/dl, ADA 24.7 U/l, erythrocytes 25,200 μl, nucleated cells 3100 μl (polymorphonuclear 39 %, lymphocytes 23 %, macrophages 38 %, and reactive mesothelial cells). Pleural fluid, sputum, and blood were collected and sent for culture.
Diuretic treatment with furosemide was intensified and empiric antibiotic coverage started with ceftriaxone. On day 4 of admission, the patient was afebrile, with negative water balance and improved pleuritic pain and dyspnea. At this time, the microbiology laboratory reported isolation of yeasts in pleural fluid, so the thoracentesis was repeated, and blood cultures were collected again. A back of the eye study was normal, and HIV serology was negative. Treatment started with fluconazole.
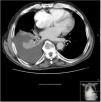
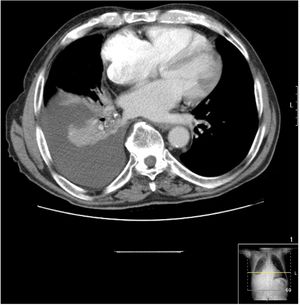
Chest CT scan revealed right free-flowing pleural effusion 5 cm thick, causing passive atelectasis of the right lower lobe (Fig. 1), with no other changes.
Blood and sputum cultures were negative. The yeast was identified as C. neoformans by 2 different microbiological methods: the VITEK system® 2 (Biomerieux, Marcy l'Etoile, France) and MALDI-TOF mass spectrometry (Bruker). The yeast was also isolated in the second pleural fluid culture. Testing for cryptococcal antigen in serum was also requested, which was negative.
Treatment was switched to voriconazole 200 mg every 12 h and thoracentesis was performed for drainage, with subsequent resolution of the effusion. Treatment continued for a month on an outpatient basis. After completion of the antifungal treatment, the patient attended a subsequent check-up in the outpatient clinic, and had returned to baseline.
C. neoformans pleuritis is a rare entity, which occurs mainly in immunosuppressed patients. In a recent review2 that included 25 cases of cryptococcal pleural effusion, 20 of the 25 patients had some type of immunosuppression; among the most common predisposing factors were HIV infection (7 cases), solid organ transplant (5 cases), or tumor disease (4 cases). In an earlier review6 of 30 cases, underlying disease was documented in 17 cases, while 10 had no predisposing factor. In that cohort, most patients with cryptococcal pleural effusion had disseminated cryptococcosis, whereas localized chest infection was more common in immunocompetent patients.6 Our patient, despite a previous diagnosis of alcoholic liver disease, presented no clinical, laboratory, or imaging data suggestive of advanced liver disease, which as we mentioned is one of the factors often associated with this infection.
Cryptococcal pleuritis can, therefore, occur in isolation, in association with lung consolidation or not, or in the context of disseminated cryptococcosis. For the diagnosis of cyptococcal pleural effusion, the organism must be isolated in a culture of pleural fluid or pleural biopsy.4 In the event of disseminated cryptococcal disease, the detection of cryptococcal antigen in the blood could be useful. Our patient had isolated effusion due to C. neoformans with no pulmonary consolidation (ruled out on CT) or dissemination to other organs (which might explain why the cryptococcal antigen in serum was negative). Although Cryptococcus was isolated in our patient from 2 cultures of pleural fluid, cultures can be sometimes negative, given the small amount of inoculum present in the pleural fluid.3,5 If the culture is negative, it may be helpful to test for cryptococcal antigen in pleural fluid (not carried out in our case), since the effusion is simply an inflammatory response to cryptococcal antigen.5
The mechanism of entry of the infection to the pleural space is usually by the pulmonary route, although the pleura could also be accessed by hematogenous spread. We believe the first route is the most likely in our case, and we speculate that his CPAP may possibly have been involved in the pathogenesis of this infection.7 In this respect, we cultured the tubing and the humidifying fluid, which were negative, although these were sampled on Day 6 of admission, after the fluid had been changed, so we could not confirm this hypothesis.
In conclusion, cryptococcal pleuritis is a rare entity which can occur in immunocompetent subjects, and one that should be taken into account in the differential diagnosis of pleural effusion in this type of patients. Given the suspicion that the source of infection in our patient could have been the CPAP, users of these devices must be made acutely aware of the importance of their appropriate use and disinfection because of the serious consequences that can result from their incorrect use.
Please cite this article as: Rodríguez-Álvarez A, Fernández-Rial Á, Pérez-López A, Pita J. Pleuritis por Cryptococcus neoformans en paciente inmunocompetente. Arch Bronconeumol. 2020;56:59–60.