Rheumatoid arthritis (RA) is a significant cause of morbidity and mortality in developed countries, with a prevalence of 0.5%–1% and an incidence of 5–50 per 100000. Nodulosis is the most common extra-articular manifestation and occurs in 25% of RA patients.1,2 Pulmonary manifestations are broad and include necrobiotic nodules, infections, drug induced lung injury, obliterative bronchiolitis, interstitial lung disease, bronchiectasis, and malignancy.
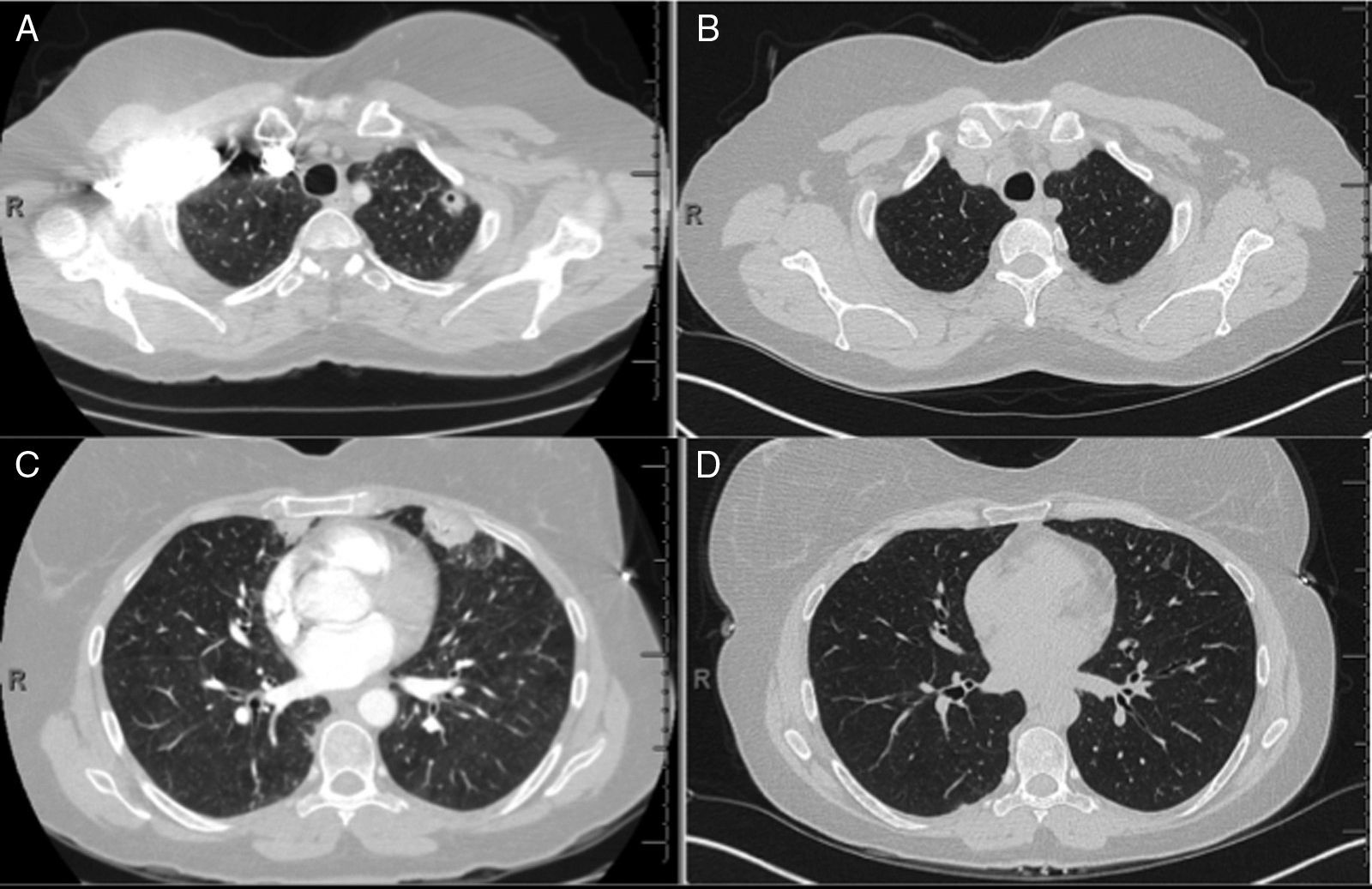
Tumor necrosis factor (TNF) is an overexpressed pro-inflammatory cytokine in RA patients, and the American College of Rheumatology has formal recommendations on the use of anti-TNF biologic agents in RA patients with poor prognostic factors. We report a case of a 50-year-old female developing multifocal nodular consolidations, with and without cavitation (Fig. 1A and C), one month after initiation of etanercept. Immunosuppressive drugs were suspended and a bronchoalveolar lavage of the left upper lobe and lingula were negative for an infectious etiology. Devoid of a confirmed infection, her RA medications were restarted, except for etanercept. Serial CT imaging demonstrated interval regression of the nodules. Radiographic changes were seen at the two-month follow up CT, and have remained unchanged at nine months (Fig. 1B and D).
CT of chest (A), initial CT demonstrating left upper lobe nodule with cavitation (B), nine month follow-up CT with resolution of left upper lobe nodule, (C) initial CT demonstrating peripheral nodule in the lingual and (D) nine month follow-up CT with resolution of peripheral nodule in the lingual.
Etanercept-associated nodular disease case reports have varied from new pulmonary nodules with histopathology typical of pulmonary RA nodules, histopathology consistent with sarcoidosis, and histopathology of lymphohistiocytic infiltrates not typical of either.3,4 Treatments for etanercept-associated PN have varied, but typically involve corticosteroids and drug withdrawal. There have also been two case reports highlighting the evolution of the nodules with continued etanercept therapy, illustrating the regression of nodules as well as stability of nodules without progression despite continued etanercept therapy.5
This case exemplifies that a recombinant human TNF receptor fusion protein may contribute to the counterintuitive formation of granulomatous disease and pulmonary nodulosis. Many mechanisms have been proposed, but the exact mechanism leading to pulmonary nodulosis is not currently known. It has been proposed that the increased size or formation of pulmonary nodules may be related to the increased size of necrotic centers, a result of the reduction of soluble TNF leading to an exaggerated or altered effect on other inflammatory pathways, or even directly related to the RA progression and not directly related to the therapeutic agent.1,4
In conclusion, we appreciate that PN is not uncommon in RA patients, but recommend that etanercept-related PN be considered as part of the differential diagnosis. Bronchoalveolar lavage should remain integral to the evaluation, but if unrevealing it would be reasonable to withhold etanercept followed by repeat imaging in 6–8 weeks. If the nodules are stable or regress, than serial imaging would be a reasonable approach. Progressive disease should be further investigated. Consideration of etanercept as the etiology of new pulmonary nodules may decrease morbidity associated with unnecessary invasive diagnostic procedures associated with nodule work up.
Please cite this article as: Zamora FD, Podgaetz E, Dincer HE. Nódulos pulmonares contraintuitivos en artritis reumatoide. Arch Bronconeumol. 2016;52:334–335.