Results supporting the use and the effectiveness of positive expiratory, pressure devices in chronic obstructive pulmonary disease (COPD) patients are still controversial. We have tested the hypothesis that adding TPEP or IPPB to standard pharmacological therapy may provide additional clinical benefit over, pharmacological therapy only in patients with severe COPD.
MethodsForty-five patients were randomised in three groups: a group was treated with IPPB, a group was treated with TPEP and a group with pharmacological therapy alone (control group).
Primary outcome measures included the measurement of scale, or questionnaire concerning dyspnoea (MRC scale); dyspnoea, cough, and sputum (BCSS) and quality of life (COPD assessment test) (CAT). Secondary outcome measures were respiratory function testing, arterial blood gas analysis and haematological examinations.
ResultsPatients in both the IPPB group and the TPEP group showed a significant improvement in two of three tests (MRC, CAT) compared to the control group. However, in the group comparison analysis for the same variables between the IPPB group and the TPEP group, we observed a significant improvement in the IPPB group (P≤.05 for MRC and P≤.01 for CAT).
The difference of action of the two techniques is evident in the results of pulmonary function testing: IPPB increases FVC, FEV1, and MIP; this reflects its capacity to increase lung volume. Also TPEP increases FVC and FEV1 (less than IPPB), and MEP, while decreasing total lung capacity and residual volume.
ConclusionsThe two techniques (IPPB and TPEP) improve significantly dyspnoea, quality of life tools and lung function in patients with severe COPD. IPPB demonstrated a greater effectiveness in improving dyspnoea and quality of life tools (MRC, CAT) than TPEP.
Los resultados que respaldan el uso y la efectividad de los dispositivos de presión espiratoria positiva en pacientes con enfermedad pulmonar obstructiva (EPOC) continúan siendo objeto de controversia. Hemos evaluado la hipótesis de que la adición de la TPEP o la IPPB a un tratamiento farmacológico estándar pueda aportar un beneficio clínico adicional respecto al tratamiento farmacológico solo en los pacientes con EPOC grave.
MétodosUn total de 45 pacientes fueron asignados aleatoriamente a los 3 grupos siguientes: un grupo fue tratado con IPPB, otro fue tratado con TPEP y un tercer grupo recibió únicamente tratamiento farmacológico (grupo de control).
Las variables de valoración principales fueron la puntuación de la escala o cuestionario relativo a la disnea (escala del MRC); la de disnea, tos y esputo (BCSS); y la de calidad de vida (test de evaluación de la EPOC) (CAT). Las variables de valoración secundarias fueron las pruebas de la función respiratoria, la gasometría arterial y los análisis hematológicos.
ResultadosTanto los pacientes del grupo de IPPB como los del grupo de TPEP mostraron una mejoría significativa en 2 de las 3 evaluaciones (MRC y CAT) en comparación con el grupo de control. Sin embargo, en el análisis de comparación de los grupos para las mismas variables en el grupo de IPPB frente al grupo de TPEP observamos una mejoría significativa en el grupo de IPPB (p≤0,05 para la escala del MRC y p≤0,01 para el CAT).
La diferencia de efecto de las 2 técnicas se pone de manifiesto en los resultados de las pruebas de la función pulmonar: la IPPB aumenta los valores de FVC, FEV1 y MIP; esto refleja su capacidad de aumentar el volumen pulmonar. Por su parte, la TPEP aumenta la FVC y el FEV1 (en menor medida que la IPPB), pero eleva la MEP, mientras que reduce la capacidad pulmonar total y el volumen residual.
ConclusionesLas 2 técnicas (IPPB y TPEP) mejoran significativamente la disnea, los instrumentos de valoración de la calidad de vida y la función pulmonar en los pacientes con una EPOC grave. La IPPB mostró una mayor efectividad en la mejora de los instrumentos de evaluación de la disnea y la calidad de vida (MRC y CAT) en comparación con la TPEP.
Chest physiotherapy by manually assisted breathing techniques is considered the gold standard for patients with chronic obstructive pulmonary diseases with normal cough reflex.1 In addition, positive expiratory pressure delivered by hand-held devices is considered a valid technique in the management of airway secretion and for enhancing expectoration.2 Several devices producing a positive expiratory pressure have been used, for example, positive expiratory pressure (PEP) mask3 or PEP bottle4 and vibratory positive expiratory pressure therapy system.5,6 However, the results supporting the use and the effectiveness of these tools are supported by little clinical evidence. Recently a new modality to deliver a low positive expiratory pressure level during spontaneous breathing called temporary positive expiratory pressure had become available.7 Intermittent positive pressure breathing (IPPB) is used in clinical practice primarily to improve lung volume and to reduce the work of breathing.17 We have tested the hypothesis that adding TPEP or IPPB to standard pharmacological therapy may provide additional clinical benefit in patients with severe to very severe COPD (Group C and D combined assessment).18
MethodsWe prospectively recruited 53 patients aged 40–80 years with severe to very severe chronic obstructive pulmonary disease (FEV1<50%) (Group C and D combined COPD assessment) admitted to the Day Hospital of the Respiratory Medicine Unit of Hospital of Sestri Levante between June 2012 and November 2012. All the patients were in a stable clinical condition (free from any acute exacerbation for at least two weeks at the time of inclusion, including no change in medication). Diagnosis and severity of COPD were confirmed using the GOLD Guidelines.18 Pulmonary function testing was performed with a computerised body plethysmograph (VMAX 20 PFT Sensor Medics, Yorba Linda, CA, USA), according to the international standards.19 The following parameters were analysed: forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), forced expiratory volume in one second/vital capacity (FEV1/FVC%), total lung capacity (TLC), residual volume (RV) and lung diffusing capacity for carbon monoxide (DLCO). Inspiratory muscle strength was assessed by measuring the maximal inspiratory mouth pressure (MIP) at RV. Expiratory muscle strength was also measured as maximal expiratory mouth pressure (MEP) at TLC. The value obtained from the best of at least three efforts was used. All the measurements were obtained in upright position.7 Patients with history of asthma, severe cardiac arrhythmias, cancer or tracheostomy, or deemed unable to perform forced expiratory manoeuvres or to use temporary positive expiratory pressure device (TPEP) or intermittent positive pressure breathing (IPPB) were excluded. Only the patients who provided informed consent were included. Patients were considered as having dropped out of the study when clinical signs of a new exacerbation occurred.
ProtocolThis was a single-blind randomised trial. A randomisation schedule was generated by a statistician not involved in the study using an online random permutation generator from http://www.randomization.com. The randomisation assignment was provided to the recruiting physicians in sealed envelopes. The patients and the investigators who carried out the study data analysis were blinded to the patients’ treatment assignments.
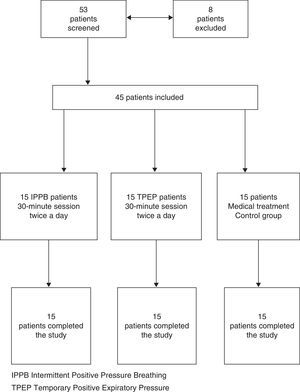
Forty-five of the 53 patients enrolled were eligible for the study (eight were excluded: seven because of inability to perform forced expiration manoeuvre and one because of coexisting history of bronchial asthma). All the patients were being treated with association of inhaled β2-agonist plus corticosteroid (28-salmeterol plus fluticasone, 17-formoterol plus budesonide) and tiotropium bromide. Five patients had chronic respiratory insufficiency treated with oxygen. The randomised patients were divided into three groups (15 patients for each group): one group was treated with IPPB, one group was treated with TPEP and one group with pharmacological therapy alone (control group) (Fig. 1, flow chart).
All the eligible patients after the randomisation were instructed by a physiotherapist on the use of temporary positive expiratory pressure (TPEP) or intermittent positive pressure breathing (IPPB) for acclimatisation in a two-hour training period in the lung laboratory before definitive inclusion in the study protocol. The TPEP device (UNIKO Medical Products Research, Legnano, Italy) delivered a fixed positive pressure (1cm H2O or 0.0977kPa) only in the expiratory phase. This increase in low pressure was created through a pulsatile flow approximately 42Hz in frequency.7 The TPEP therapy was delivered by a use-specific mouthpiece.
The IPPB device (ALPHA 200C, Air Liquide) delivered an inspiratory pressure that was gradually increased to the highest tolerated value (up to 40cm H2O). Respiratory rate, inspiratory flow (from 20 to 60l/min) and end-inspiratory trigger were set to maximise patient comfort. The IPPB therapy was also delivered by a use-specific mouthpiece.
Both treatments lasted 30min per session and were given twice daily (morning and late afternoon). The duration of each treatment was fifteen days and the treatment was administered five days per week. The study was carried out according to the principles of the Declaration of Helsinki and approved by Institutional Ethics Committee of ASL 4 Chiavarese, Chiavari, Italy; all patients provided written informed consent before beginning the study. The study was registered as Chi CTR-TRC-12002178 at www.chictr.org.
Measurements and OutcomesAt enrolment, patients’ anthropometric and physiological characteristics were recorded. Primary outcome measures included dyspnoea, cough and sputum scales, as well as daily life activity evaluations. Secondary outcome measures were respiratory function testing (FVC, FEV1, FEV1/FVC%, TLC, RV, DLCO, MIP, MEP), arterial blood gas analysis (paO2, paCO2, pH) and haematological examinations (white and red cell counts, C reactive protein, γ-globulins). Dyspnoea, cough and sputum and daily life activities were measured with the Breathlessness, Cough and Sputum Scale (BCSS),20 COPD Assessment Test (CAT)21,22 and the Medical Research Council (MRC) Dyspnoea Scale.
Dynamic and static volumes were expressed as a percentage of their predicted value. Maximal inspiratory and expiratory pressure (MIP/MEP) was expressed as absolute values (kPa). BCSS and CAT were recorded each day to evaluate the daily variations in dyspnoea and perceived sensation of bronchial encumbrance and recorded as additional study outcomes.
StatisticsClinical data were expressed as count, percentage (%) and mean (±standard deviation, SD). We initially calculated the difference between each treatment and control group. The difference between the two treatment groups (IPPB and TPEP) and the control group was evaluated using a univariate (covariance) analysis. Subsequently the difference between the two treatment groups (IPPB and TPEP) was analysed using covariance analysis; P≤.05 was considered statistically significant. Data analysis was made with statistics software R-Project version 2.13.2.
ResultsParticipantsAll forty-five enrolled patients completed the study. Twenty-six were males (nine in the TPEP group, nine in the IPPB group and eight in the control group); nineteen were females (six in the TPEP group, six in the IPPB group and seven in the control group). The average age was 73±6 years in the TPEP group, 70±9 years in the IPPB group and 70±6 years in the control group.
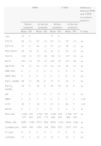
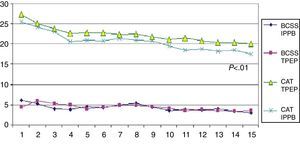
Primary OutcomesPatients in both the IPPB group and the TPEP group showed a significant improvement in the evaluation of dyspnoea (MRC) and quality of life assessment (CAT) compared to the control group (Table 1). However, in the group comparison analysis for the same variables between the IPPB group and the TPEP group, we observed a significant improvement in the IPPB group (P≤.05 for MRC and P≤.01 for CAT) (Table 2). Fig. 2 shows the daily trend of CAT and BCSS tests during the study period.
Changes in Lung Volumes, Gas Exchange, Dyspnoea and Quality of Life Scales, and Muscle Strength and Biochemical Parameters Before and After Treatment in the Three Groups.
| Control | IPPB | T-PEP | Difference between the two methods and control group (covariance analysis) | |||||||||||
| Before treatment | At the last treatment | Before treatment | At the last treatment | Before treatment | At the last treatment | IPPB | T-PEP | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | P-value | P-value | |
| Age | 70 | 6 | 70 | 9 | 73 | 6 | ||||||||
| FVC% | 63 | 11 | 57 | 14 | 58 | 11 | 67 | 12 | 63 | 11 | 70 | 12 | <.001 | <.001 |
| FEV1% | 30 | 7 | 28 | 8 | 37 | 9 | 44 | 11 | 31 | 11 | 38 | 11 | <.01 | <.05 |
| FEV1/FVC | 37 | 5 | 37 | 5 | 48 | 10 | 51 | 12 | 38 | 6 | 43 | 12 | ns | ns |
| TLC% | 130 | 23 | 139 | 20 | 128 | 31 | 117 | 28 | 154 | 31 | 127 | 19 | <.05 | <.001 |
| RV% | 208 | 68 | 212 | 62 | 203 | 34 | 171 | 33 | 287 | 75 | 217 | 46 | ns | <.01 |
| DLCO% | 49 | 18 | 46 | 16 | 54 | 11 | 55 | 12 | 54 | 22 | 56 | 21 | ns | ns |
| MIP, kPa | 5 | 1 | 4 | 1 | 4 | 2 | 5 | 2 | 3 | 2 | 4 | 2 | <.01 | ns |
| MEP, kPa | 5 | 1 | 5 | 1 | 5 | 2 | 6 | 2 | 4 | 2 | 6 | 4 | ns | <0.05 |
| PaO2, mmHg | 64 | 8 | 63 | 8 | 66 | 11 | 68 | 6 | 72 | 8 | 74 | 7 | ns | ns |
| PaCO2, mmHg | 46 | 9 | 46 | 9 | 48 | 6 | 48 | 6 | 45 | 10 | 43 | 9 | ns | ns |
| pH | 7 | 0 | 7 | 0 | 7 | 0 | 7 | 0 | 7 | 0 | 7 | 0 | ns | ns |
| MRC | 3 | 1 | 4 | 1 | 4 | 0 | 3 | 1 | 4 | 0 | 4 | 1 | <.001 | <.05 |
| CAT | 27 | 6 | 27 | 6 | 26 | 7 | 18 | 7 | 27 | 6 | 20 | 7 | <.001 | <.05 |
| BCSS | 5 | 2 | 5 | 2 | 6 | 2 | 3 | 1 | 6 | 2 | 4 | 2 | ns | ns |
| Red cells | 4507250 | 281847 | 3938250 | 1420499 | 4644875 | 525462 | 4444625 | 301775 | 4485000 | 365865 | 4548000 | 397043 | ns | ns |
| White cells | 8714 | 1616 | 8795 | 1764 | 8081 | 1100 | 8571 | 689 | 8670 | 1254 | 8344 | 1461 | ns | ns |
| Lymphocytes | 2959 | 546 | 2969 | 634 | 1849 | 340 | 1918 | 248 | 2691 | 925 | 2435 | 831 | ns | ns |
| C-r prot | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ns | ns |
| γ-Globul | 12 | 1 | 13 | 1 | 13 | 2 | 12 | 2 | 12 | 1 | 12 | 1 | ns | ns |
FVC, forced vital capacity; BCSS, breathless cough sputum score; DLCO, lung diffusing for carbon monoxide; FEV1, forced expiratory volume 1s; PaO2, oxygen arterial pressure; C-r prot, C reactive protein; FEV1/FVC, ratio; PaCO2, carbon dioxide arterial pressure; γ-globul, gamma globulins; TLC, total lung capacity; MRC, Medical Research Council scale; MIP, maximal inspiratory pressure; RV, residual volume; CAT, COPD assessment test; MEP, maximal expiratory pressure; IPPB, intermittent positive pressure breathing; T-PEP, temporary positive expiratory pressure; ns, not significant.
Changes in Lung Volumes, Gas Exchange, Dyspnoea and Quality of Life Scales, and Muscle Strength and Biochemical Parameters Before and After Treatment in the IPPB and TPEP Groups.
| IPPB | T-PEP | Difference between IPPB and T-PEP (covariance analysis) | |||||||
| Before treatment | At the last treatment | Before treatment | At the last treatment | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | P-value | |
| Age | 70 | 9 | 73 | 6 | |||||
| FVC% | 58 | 11 | 67 | 12 | 63 | 11 | 70 | 12 | ns |
| FEV1% | 37 | 9 | 44 | 11 | 31 | 11 | 38 | 11 | ns |
| FEV1/FVC | 48 | 10 | 51 | 12 | 38 | 6 | 43 | 12 | ns |
| TLC% | 128 | 31 | 117 | 28 | 154 | 31 | 127 | 19 | ns |
| RV% | 203 | 34 | 171 | 33 | 287 | 75 | 217 | 46 | ns |
| DLCO% | 54 | 11 | 55 | 12 | 54 | 22 | 56 | 21 | ns |
| MIP, kPa | 4 | 2 | 5 | 2 | 3 | 2 | 4 | 2 | ns |
| MEP, kPa | 5 | 2 | 6 | 2 | 4 | 2 | 6 | 4 | ns |
| PaO2, mmHg | 66 | 11 | 68 | 6 | 72 | 8 | 74 | 7 | ns |
| PaCO2, mmHg | 48 | 6 | 48 | 6 | 45 | 10 | 43 | 9 | ns |
| pH | 7 | 0 | 7 | 0 | 7 | 0 | 7 | 0 | ns |
| MRC | 4 | 0 | 3 | 1 | 4 | 0 | 4 | 1 | <.05 |
| CAT | 26 | 7 | 18 | 7 | 27 | 6 | 20 | 7 | <.01 |
| BCSS | 6 | 2 | 3 | 1 | 6 | 2 | 4 | 2 | ns |
| Red cells | 4644875 | 525462 | 4444625 | 301775 | 4485000 | 365865 | 4548000 | 397043 | ns |
| White cells | 8081 | 1100 | 8571 | 689 | 8670 | 1254 | 8344 | 1461 | ns |
| Lymphocytes | 1849 | 340 | 1918 | 248 | 2691 | 925 | 2435 | 831 | ns |
| C-r prot | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ns |
| γ-Globul | 13 | 2 | 12 | 2 | 12 | 1 | 12 | 1 | ns |
FVC, forced vital capacity; BCSS, breathless cough sputum score; DLCO, lung diffusing for carbon monoxide; FEV1, forced expiratory volume 1s; PaO2, oxygen arterial pressure; C-r prot, C reactive protein; FEV1/FVC, ratio; PaCO2, carbon dioxide arterial pressure; γ-globul, gamma globulins; TLC, total lung capacity; MRC, Medical Research Council scale; MIP, maximal inspiratory pressure; RV, residual volume; CAT, COPD assessment test; MEP, maximal expiratory pressure; IPPB, intermittent positive pressure breathing; TPEP, temporary positive expiratory pressure; ns, not significant.
No significant changes were observed for the blood biochemical measurements, nor were significant changes found in arterial blood gases. As shown in Table 1, there was a significant increase at the end of the treatment in forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and maximal inspiratory pressure (MIP) and a decrease in total lung capacity (TLC) in the IPPB group compared to the control group. FVC, FEV1 and maximal expiratory pressure (MEP) increased significantly and TLC and residual volume (RV) decreased in the TPEP group compared to the control group (Table 1). No differences were observed in the comparison analysis between the IPPB and TPEP groups (Table 2).
DiscussionRecently, a new modality to mechanically deliver a low positive expiratory pressure at the mouth during spontaneous breathing, called temporary positive expiratory pressure, was introduced to assist patients with chronic obstructive pulmonary disease. This technique produces a 1cm H2O increase in airway pressure throughout the respiratory cycle until immediately before expiration. The level of applied pressure with temporary positive expiratory pressure is considerably lower than the level normally used [(5–15cm H2O) by previous devices]. IPPB is an established therapeutic device used in several fields of pulmonary rehabilitation.8 It uses a mechanical ventilator to deliver a controlled pressure of a gas to assist in ventilation or expansion of the lungs, thereby providing an increased tidal volume. IPPB can include pressure- and time-limited ventilation, as well as pressure-, time- and flow-cycled ventilation. IPPB machines are also used for the delivery of aerosol medications.9 IPPB remains a technique used to provide short-term or intermittent mechanical ventilation for the purpose of increasing lung expansion, delivering aerosol medication, and/or assisting in ventilation.10 Few studies have been published regarding the use of IPPB in COPD patients: one of the oldest of these demonstrated little advantage in aerosol therapy.11 A second study showed no advantage in adding IPPB to traditional chest physiotherapy.12 The next, a multicentre study, which compared intermittent positive pressure breathing (IPPB) therapy with compressor nebuliser therapy in 985 ambulatory patients showed no advantage of IPPB over compressor nebuliser therapy.13 Since the late 1980s, the use of IPPB was progressively abandoned for several reasons highlighted by Duffy and Furley.14 The device was continued to be used in patients with restrictive pulmonary diseases (e.g. neuromuscular diseases). More recently two studies have been published in which IPPB increases pulmonary functional parameters (vital capacity, total lung capacity, functional residual capacity, as well as maximal inspiratory and expiratory pressure) along with global ventilation in patients with neuromuscular diseases.15,16 The use of positive expiratory pressure to improve lung volume is well documented in the literature; physiotherapists use this tool in patients with low lung volumes to improve ventilation and gas exchange. IPPB is used in clinical practice primarily to improve lung volume and to reduce the work of breathing.17
Preliminary results show that an expiratory pressure of ≤1cm H2O applied for a fraction of the expiratory phase may improve the distribution of alveolar ventilation.7 Only one study has been published on TPEP, and this paper demonstrated its efficacy in improving lung volumes and speeding the clearance of bronchial encumbrance after short-term (10 days) treatment.7
In this study, different primary outcomes were considered (inspiratory oxygen fraction ratio – PaO2/FiO2), as were the secondary outcomes of pulmonary function parameters, and daily variations of sputum production, density, purulence and bronchial encumbrance evaluated on a visual analogue scale. In the group analysis of the pre-to-post change for the same variable, only inspiratory capacity was more significantly increased and sputum purulence reduced in the group treated with TPEP. The methodology (TPEP was added to the traditional manually assisted breathing techniques), the outcomes and the results make it difficult to compare our study with the previous one: the increase in inspiratory capacity (IC) suggests a reduction in pulmonary hyperinflation as we observed in our patients [insignificant reduction of total lung capacity (TLC) and residual volume (RV)]. In an additional study, TPEP was used for 30min twice per day for a short period (5 days) in preparation for major abdominal surgery.23 We also found a non-randomised, controlled study (written in Italian) (in which data from former patients were inserted as control group) showing an improvement in PaO2 and functional lung parameters after using TPEP for 28 days.24 The IPPB was widely used in post-surgery patients where it improves lung volume and enhances the effectiveness of cough.25 In acute quadriplegic patients it increases tidal volume and forced vital capacity, allowing lung inflation and ventilation, thus reducing chest wall and pulmonary compliance.17 Apart from the use of IPPB as a tool in delivering aerosols to the bronchial tree, there are no studies that confirm the usefulness of IPPB in COPD patients. One of the reasons is that IPPB requires high levels of operator skill in manipulating the settings appropriately.17 Actually the latest IPPB (Alpha 200C, Air Liquide) (see Fig. 3) works similarly to a bilevel ventilator (BIPAP), but remains a tool in chest physiotherapy. It is easier to set up and may be used with a mouthpiece or with a mask.
Our study is the first to consider only COPD patients and in this sense it could be considered a pilot study. (The previous study enrolled also patients with bronchiectasis.)7 This is the first clinical trial to verify the effectiveness of IPPB in severe COPD patients and seeks to confirm the effectiveness of TPEP. The difference of action of the two different techniques is evident in the results of pulmonary function testing: IPPB increases FVC, FEV1, and MIP; this reflects its capacity to increase lung volume and enhance effectiveness of the cough,15–17 but it does not decrease total lung capacity and residual volume. TPEP increases FVC and FEV1 (less than IPPB), and also MEP, while decreasing total lung capacity (TLC) and residual volume (RV). The latter observation is important because it could functionally explain the suggested mechanism of action of TPEP on small bronchioles producing an improvement in alveolar ventilation obtained with only 1cm H2O throughout the respiratory cycle.23
LimitationsAlthough this study reports some interesting new findings on the use of two positive expiratory pressure techniques and was done in a strictly selected population and a limited number of patients with a randomised controlled design, we are aware of some limitations in our study and the limited statistical power of our samples.
We did not study more complex functional outcomes (e.g. six-minute walk test) to verify whether the recorded improvements in lung functions and quality of life might be related to a better general condition linked to daily activities.
ConclusionsThe two techniques (IPPB and TPEP) significantly improve dyspnoea, quality of life tools and lung function in patients with severe COPD. IPPB demonstrated a greater effectiveness in the improvement of dyspnoea (MRC) and quality of life (CAT) tools than TPEP.
Conflict of InterestThis study was carried out independently from the manufacturers and the authors have no conflict of interest to declare.
The authors thank Dr. Norma Landucci for helping in data collection, Dr. Maura Ferrari-Bravo for helping in statistical analysis and Dr. Cornelius Barlascini for valuable help in editing and revising the manuscript.
Please cite this article as: Nicolini A, Mollar E, Grecchi B, Landucci N. Comparación de la respiración con presión positiva intermitente y la presión espiratoria positiva temporal en pacientes con enfermedad pulmonar obstructiva crónica grave. Arch Bronconeumol. 2014;50:18–24.