The PLATINO baseline study, conducted from 2003 to 2005 in five Latin American cities (São Paulo, Mexico City, Montevideo, Santiago, Caracas), showed a high prevalence of chronic obstructive pulmonary disease (COPD).
Methods/designA follow-up study was conducted in three out of the five centers (Montevideo, Santiago, and São Paulo) after a period of 5, 6 and 9years, respectively, aimed at verifying the stability of the COPD diagnosis over time, the evolution of the disease in terms of survival, morbidity and respiratory function, and the analyses of inflammatory and genetic biomarkers in the blood. Some questions were added to the original questionnaire and death certificates were obtained from the national official registries.
ResultsThe fieldwork has been concluded in the three centers. From the original samples in the PLATINO study phase i, we were able to locate and interview 85.6% of patients in Montevideo, 84.7% in Santiago and 77.7% in São Paulo. Individuals who could not be located had higher education levels in Brazil, and were more likely to be current smokers in Santiago and São Paulo than in Montevideo. The overall quality of spirometries was ≥80% according to American Thoracic Society criteria. The number of deaths was 71 (Montevideo), 95 (Santiago) and 135 (São Paulo), with death certificates obtained from the national mortality registries for 76.1%, 88.3% and 91.8% of cases in Montevideo, Santiago and São Paulo, respectively.
ConclusionsThis study shows that it is possible to perform population-based longitudinal studies in Latin American with high follow-up rates and high-quality spirometry data. The adequacy of national mortality registries varies among centers in Latin America.
El estudio basal del PLATINO, llevado a cabo entre 2003 y 2005 en 5ciudades latinoamericanas (São Paulo, Ciudad de México, Montevideo, Santiago, Caracas), mostró una prevalencia elevada de la enfermedad pulmonar obstructiva crónica (EPOC).
Métodos/diseñoSe llevó a cabo un estudio de seguimiento en 3 de los 5 centros (Montevideo, Santiago y São Paulo) después de un periodo de 5, 6 y 9años, respectivamente, con el objetivo de verificar la estabilidad del diagnóstico de EPOC a lo largo del tiempo, la evolución de la enfermedad en cuanto a supervivencia, morbilidad y función respiratoria, y análisis de los biomarcadores genéticos e inflamatorios en sangre. Se añadieron algunas preguntas adicionales al cuestionario original y se obtuvieron los certificados de defunción a partir de los registros oficiales nacionales.
ResultadosEl trabajo de campo se ha completado en los 3 centros. De las muestras originales de la fasei del PLATINO pudimos localizar y entrevistar al 85,6% en Montevideo, al 84,7% en Santiago y al 77,7% en São Paulo. Los individuos no localizados se caracterizaban por un mayor nivel de estudios en Brasil y era más probable que fueran fumadores actuales en Santiago y São Paulo que en Montevideo. La calidad global de las espirometrías fue ≥ 80% según los criterios de la American Thoracic Society. El número de muertes fue de 71 (Montevideo), 95 (Santiago) y 135 (São Paulo), y se obtuvieron los certificados de defunción a partir de los registros de mortalidad nacionales del 76,1, del 88,3 y del 91,8% de los casos en Montevideo, Santiago y São Paulo, respectivamente.
ConclusionesEste estudio muestra que es posible realizar estudios longitudinales de base poblacional en Latinoamérica, con tasas de seguimiento elevadas y una alta calidad de los datos de espirometría. La idoneidad de los registros de mortalidad nacionales varía en los distintos centros de Latinoamérica.
Studies on the prevalence of COPD in Latin America (LA) are scarce; the PLATINO project was a multicenter study carried out in five centers in LA, filling an important gap of knowledge in this area.1
The original aim of the PLATINO study, launched in 2002, was to describe the epidemiology of COPD in five major LA cities: São Paulo (Brazil), Santiago (Chile), Mexico City (Mexico), Montevideo (Uruguay), and Caracas (Venezuela), among adults aged 40 years or more, since subjects less than 40 years of age have low prevalence of COPD.2 A secondary objective was to evaluate the prevalence of self-reported medical co-morbidities that can occur quite often with COPD, such as cardiovascular diseases and lung cancer.
These sites represent the various different geographical areas of LA and the largest metropolitan area in each participating country. The study was conducted from 2002 to 2004 as an initiative from the Associación Latinoamericana del Tórax (ALAT).
The PLATINO baseline study revealed a high prevalence of COPD in Latin America based on the fixed ratio criteria (FEV1/FVC <70% post bronchodilator), figures previously unknown for Latin America.3,4
Some of the most relevant aspects of the PLATINO baseline study were: (a) the fact that it was a population-based study and that most of the studies on COPD in the literature are carried out among selected COPD patients; (b) the high response rate in all centers; and (c) the high quality of the spirometries.
The detailed methods of the study are published elsewhere.5
However, this was a cross sectional study comprising of a visit at one point in time and this does not allow us to analyze temporality and some outcomes of interest in COPD. Therefore, after 5 years, it was decided that a follow-up of the study in some centers from the original project should be carried out.
Several issues were raised concerning the difficulties of following people in LA, such as the high level of migration and mobility, the lack of official registries to locate the same subjects, the possibility of death with subjects aged 40 years and older since the baseline study, and the adequacy of the information on vital statistics for certifying death. Another potential important problem was the increased violence in LA, which could be a limitation for visiting households in some areas of the cities. Funding was obtained to repeat the study in three centers out of the five; they were chosen according to the limitations listed above and to the prevalence level of COPD found in the baseline.
The first follow-up visit took place in the city of Montevideo (5 years after the baseline), given that this center presented the highest prevalence of COPD in the PLATINO baseline study (19.7%; 95% CI 17.2–22.1), it is a small sized city, and it has very low mobility level among people. Santiago was chosen as the second center for the follow-up, as it occupied the second place in the ranking of prevalence of COPD (16.9%; 95% CI 14.7–19.1), it presented the highest prevalence of smoking in the baseline study (38.6%), and because it is considered a safe place in LA. Finally, the follow-up was done in a third center (Sao Paulo), since the city was ranked as the third one on COPD prevalence (15.8%; 95% CI 13.5–18.1).4 The time interval between the two visits in each of the centers was different, varying from 5, 6 to 9 years in Montevideo, Santiago, and Sao Paulo (SP), respectively. The main reason for the different times between both phases of the study was finding the resources to fund the project. The general objectives of the follow-up study were: (a) to evaluate the natural history of COPD in the same sample of subjects from the original PLATINO project; (b) to validate the reliability of original diagnosis of COPD; (c) to describe the natural history of COPD in terms of survival, morbidity, occupational history, respiratory function, respiratory symptoms, exacerbations, general health status, physical activity limitation, management, hospitalization, absenteeism, nutrition, among others; and (d) to obtain blood samples for analyzing biomarkers.
The present paper describes: (a) the methods used in a population-based longitudinal study in Latin America; (b) the preliminary results, such as follow-up rates, spirometry quality control, information on the deaths occurring during the period; (c) the procedures for collecting and storing blood samples; and (d) the main limitations for carrying out such studies in Latin America.
MethodsThe design of the follow-up visit was cross sectional and the target population was the same one that had been investigated and that had performed spirometry; as we ended up with two visits over time for the same population, we can say that the design of the overall study is a cohort design.
Each census tract and household visited in the baseline study was contacted again and the same procedures were employed as in the PLATINO baseline study. All eligible adults from the original sample answered a questionnaire and performed the same procedures as before.5
The previous and new variables collected in both phases of the PLATINO study are listed in Table 1. Details of the questionnaires can be found at the PLATINO website.1
Variables Collected in Each Center at Baseline and Follow-up. The PLATINO Study.
| Variables | Montevideo (Uruguay) | Santiago (Chile) | Sao Paulo (Brazil) | |||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Demographic and socioeconomic | X | X | X | X | X | X |
| Symptoms and respiratory diseases | X | X | X | X | X | X |
| Management of disease and medicine use | X | X | X | X | X | X |
| Smoking | X | X | X | X | X | X |
| Fagerstrom scale14 | – | X | – | X | – | X |
| Occupational exposure to dust | X | X | X | X | X | X |
| Comorbidities (heart diseases, hypertension, diabetes and lung cancer) | X | X | X | X | X | X |
| Passive smoking | X | X | X | X | X | X |
| Quality of life (SF-12)13 | X | X | X | X | X | X |
| Economic impact | X | X | X | X | X | X |
| Leisure-time activities | X | X | X | X | X | X |
| Intra-household pollution | X | X | X | X | X | X |
| Physical Activity33 | – | X | X | – | X | |
| Sleep and insomnia | X | X | X | X | – | X |
| Oxygen use | X | X | X | X | X | X |
| Beck inventory (depression scale)16 | – | X | – | X | – | X |
| Anthropometry | X | X | X | X | X | X |
| Blood pressure | – | – | – | X | – | – |
| Oxygen saturation | X | X | X | X | – | – |
| Spirometry | X | X | X | X | X | X |
Blood samples were collected for the first time in the second phase of the PLATINO study; therefore, it is a cross sectional rather than longitudinal data. These tests can be used as the baseline information for future follow-ups. After contacting the eligible subjects in each of the households the interviewers asked them if they would agree to have a blood sample collected; if the subjects agreed, the clinical laboratory was contacted and took charge of the arrangements to go to each house and obtain the blood samples. Fasting blood samples (8h) were taken from the subjects to measure serum and genetic biomarkers6–8; the time between the collection of the blood and the arrival at the laboratory was on average 3h (in the meantime, samples were kept at 4°C). The following procedure was followed for DNA extraction9: a sample of 5mL of peripheral venous blood was collected in EDTA vials and transferred to 15mL conical tubes. A solution of red blood cell lysis (9–10mL) (NH2CL 114mM, NH4HCO3 1mM) was added to the venous blood and the tube was incubated for 30min at 4°C. Afterwards, the material was centrifuged and the leukocyte button was recovered and stored at −80°C. Serum was obtained from 15mL of peripheral blood collected in vials containing coagulation accelerator and frozen at −80°C for analysis of serum biomarkers.
According to the literature, some possible biomarkers to be explored in the future include: chemoattractants (Interferon gamma-induced protein 10 [IP-10], Interferon-inducible T-cell alpha chemoattractant [iTAC], eotaxin-2 [Eot2], myeloid progenitor inhibitory factor-1 [MPIF 1], monocyte chemotactic protein-1 [MCP1], macrophage inflammatory protein 1a and 1b [MIP1a and MIP1b], interleukin-8 [IL-8], thymus and activation regulated chemokine [TARC]), inflammation (inteleukin-15 [IL-15], interleukin-1 receptor antagonist [IL-1ra], interleukin-17 [IL-17], tumor necrosis factor-alpha/TNF receptor 1 [TNFa/TNF R1], interferon gamma [IFNg], interleukin-12, p40 subunit [IL_12P40], interleukin 2 receptor, gamma [IL-2Rg], interleukin-6 [IL-6], interleukin-8 [IL-8]), destruction and repair (tumor growth factor alpha [TGFa], vascular endotelial growth factor [VEGF], androgen receptor [AR], brain-derived neurotrophic factor [BDNF], beta-nerve growth factor [bNGF], matrix metallopeptidase 9 [MMP9], tissue inhibitor of metalloproteinases [TIMP1]) novel markers (plasminogen activator inhibitor-2 [PAI_II], Prolactin hormone) and lung specific markers (desmosine, surfactant pulmonary protein D [SPD], clara cell secretory protein [CC-16] and chemokine (C-C motif) ligand-8 [CCL-18]).10,11 Blood samples will be kept frozen at −80°C in each of the centers and will be analyzed after obtaining funding for it.
MortalityDuring the visits of each household of the PLATINO study baseline, the vital status of the subjects who comprised the original sample of the study was asked. If someone at that house informed the interviewer that the subject(s) from the original study had died, the information was transferred to a team member responsible for getting more information about the death. That person had to contact the national official mortality registry and to assess the information from the official death certificates. This was done in each of the three centers.
The original protocol aimed to obtain data from the hospital for those who died during the hospitalization; due to logistic reasons and also due to the inadequacy of the information on the hospital registry observed in the first site of the study (Montevideo) this was not carried out in the other two sites (Santiago and Sao Paulo).
Information about the death was certified at the official mortality registry of each center and the death certificates were checked. In Uruguay, the registry was not electronic and the certificates had to be localized among all the other death certificates. In Chile and in Brazil, there was an electronic registry and it was easier to locate the death certificates.
All the information on the certificates was transcribed on an Excel spreadsheet exactly as they were written in the certificates. Name of the subject, birth date, death date, basic cause of death and all the other contributory death causes were recorded.
After the end of the follow-up study in the three centers, we elected an expert in mortality registry (from Brazil) and this person reviewed all the death certificates; he certified the basic cause of death based on the information on the death certificates and he ranked the contributory causes of death. The information on the death certificates from Uruguay was very poor and inadequate; quite often the cause of death written in the certificates was “cardiorespiratory arrest” or “natural death”.
After a careful evaluation of the death certificates of the three centers it was decided by the coordination of the study and by the expert on mortality registries that it was impossible to rank the death causes occurred in Uruguay. The only information obtained from the death certificates in Uruguay was the basic cause of death from those certificates considered as having the essential information for certifying the basic cause of death.
Quality Control for Questionnaires, Spirometry and AnthropometryThe same procedures described for the quality control in the PLATINO baseline were adopted in the follow-up study; details of the procedures can be found elsewhere.5
QuestionnaireThe questionnaire was a composite that included sections of the following questionnaires: ATS/DLD12 and ECRHS II; the SF-1213 was also added to assess overall health status. Some other questions and specific instruments were added to the original questionnaire, such as: questions about the new legislation anti-smoking in all sites and the Fagerstrom scale14; the two other instruments added to the original questionnaire were the Baecke questionnaire for measuring physical activity15 and the Beck Inventory Depression for measuring depression.16
SpirometryA portable, battery-operated, ultrasound transit-time based spirometer (Easy-One from NDD™) was used. The spirometers had their calibration checked daily with a 3-liter syringe before being used in the field. The spirometers stored up to 400 test results in a memory chip which was downloaded regularly. The initial evaluation performed immediately after a short questionnaire established whether the subject was eligible for this procedure (presence in the last three months of thoracic or abdominal surgery, heart attack, eye surgery or retinal detachment, hospitalization for any heart problem, current treatment for tuberculosis, self-reported pregnancy or pulse rate above 120 or below 60 beats/min) and after anthropometric examination was completed. Subjects then performed a number of attempts until these resulted in three ATS acceptable maneuvers, with FVC and FEV1 reproducible to 150ml.17 A bronchodilator (salbutamol 200mcg) was then administered by inhalation, and the test was repeated 15min later, with the same criteria. All spirometric examinations were carried out with the subject seated, wearing a nose clip and a disposable mouthpiece.18
Height MeasurementA portable Seca® stadiometer (precision 0.1cm) was used for measuring height, using the technique recommended by Lohman et al.19 Subjects did not wear shoes and they were asked to stand with their feet in contact with the base of the stadiometer and to keep their heads straight in the Francfort plane while their height was checked.
WeightAn electronic Tanita® weight scale (precision 200g) was used. Subjects were weighed without shoes and wearing light clothes.
Waist CircumferenceAn inextensible Fiberglass® tape (precision 0.1cm) was used. Firstly the interviewers identified the midpoint between the last rib and the iliac crest; then the tape was placed horizontally around the waist over the midpoint, neither too tight nor too loose.20
The measurements of height, weight and waist circumference were carried out twice on each subject, and the average value was used.
Ethical ConsiderationsEthical approval was obtained from the ethical committee of each center. The consent form approved by the ethical committees mentioned the administration of a questionnaire, the use of a bronchodilator in two moments, the performance of a spirometry test and a blood collection for future analysis of genetic and inflammatory biomarkers.
Only subjects who agreed to participate in the study and signed the informed consent were eligible. Those who did not agree to be part of the research or who did not sign the consent form were considered losses. The results of spirometries were sent to all subjects and those who had COPD or any abnormality in the spirometry were offered the possibility of attending a medical consultation in a clinical center.
Processing of DataBefore sending the original questionnaires to the Coordinating Center in Pelotas, Southern Brazil, all questionnaires were photocopied and kept in each center. In the Coordinating Center, all questionnaires were reviewed and open answers were coded with the same codes as from the baseline study. Data were entered twice in the Epidata 3.121 and the two entries were compared. Any difference detected between the two datasets was checked in the original questionnaire and corrections were made; after all data were cleaned, they were transferred to another software (STATA). Spirometry results were cleaned and edited in Mexico City, and then sent to the Coordinating Center and linked to the questionnaire database.22
AnalysisAnalyses were carried out using the STATA program. These include descriptive analyses of the outcome variables in both visits of PLATINO. Also, samples were compared in terms of demographic, socioeconomic, behavioral and nutritional variables. The second set of analyses included the calculation of the prevalence of COPD in the PLATINO follow-up according to COPD status in the PLATINO baseline, and according to several COPD criteria. Third, we analyzed mortality using Cox models as time to death differs among the three countries. All analyses took into account the cluster sampling procedure23 and adjusted for the difference in time according to each center.
ResultsThe fieldwork was done sequentially and, therefore, we had different interval times between the two visits for each center. Table 2 shows the mean time of follow-up for each center, of which Sao Paulo took the longest. The number of deaths, refusals and losses was higher in Sao Paulo than in the other centers; nevertheless, the overall response rate for spirometries (95%) was nearly the same among the three centers. The overall response rate for the blood samples was 75.3% in Montevideo, 82.7% in Santiago and 60% in Sao Paulo (Table 2).
Baseline and Follow-up Samples According to Center. The PLATINO Study.
| Montevideo (Uruguay) | Santiago (Chile) | Sao Paulo (Brazil) | |
| No. | No. | No. | |
| Time to follow-up, years (mean [SD]) | 4.9 (0.1) | 6.5 (0.2) | 8.8 (0.1) |
| Original sample size at baseline | 943 | 1208 | 1000 |
| Spirometries performed at baseline (eligible subjects for follow-up) | 885 | 1173 | 963 |
| Located subjects | 872 | 1118 | 943 |
| Deaths | 71 | 95 | 135 |
| Refusals | 76 | 63 | 141 |
| Losses | 35 | 62 | 54 |
| Subjects interviewed | 687 | 898 | 613 |
| Spirometries performed at follow-up | 683 | 858 | 596 |
| Overall response rates of interviews (%) | 85.6 | 84.7 | 77.7 |
| Overall response rates including spirometries (%) | 99.4 | 95.5 | 97.2 |
| Overall response rates including blood samples (%) | 75.3 | 84.5 | 60.0 |
The follow-up rates according to key baseline characteristics by country are shown in Table 3. In the period of 5–9 years, the percentage of follow-up rates did not vary according to sex, age, skin color, family history of chronic bronchitis, emphysema or COPD, passive smoking, waist circumference, and Body Mass Index (BMI). Subjects from lower schooling levels were more likely to be located in the city of Sao Paulo, although there was no difference compared to the other sites. With regard to smoking status, there was a higher percentage of follow-up rates for non-smoking subjects compared to smokers (88.5% vs 80.6%) in Santiago, and a higher percentage of traced subjects among ex-smokers (82.0%) in Sao Paulo.
Follow-up Rates According to Key Baseline Characteristics by Country. The PLATINO Study.
| Variables | Uruguay (Montevideo) | Chile (Santiago) | Brazil (São Paulo) | |||
| Eligible subjects (No.=885) | Locateda | Eligible subjects (No.=1173) | Locateda | Eligible subjects (No.=963) | Locateda | |
| No. | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Sex | P=.458 | P=.166 | P=.157 | |||
| Male | 361 | 313 (86.7) | 454 | 376 (82.8) | 427 | 327 (76.6) |
| Female | 524 | 445 (84.9) | 719 | 617 (85.8) | 536 | 389 (72.6) |
| Age (years) | P=.495 | P=.372 | P=.095 | |||
| 40–49 | 237 | 205 (86.5) | 397 | 329 (82.9) | 383 | 271 (70.8) |
| 50–59 | 237 | 207 (87.3) | 377 | 319 (84.6) | 308 | 233 (75.7) |
| ≥60 | 411 | 346 (84.2) | 399 | 345 (86.5) | 272 | 212 (77.9) |
| Skin color/ethnicity | P=.378 | P=.038 | P=.836 | |||
| White | 793 | 676 (85.3) | 815 | 688 (84.4) | 556 | 410 (73.7) |
| Mulatto | 52 | 49 (94.2) | 287 | 249 (86.8) | 265 | 196 (74.0) |
| Black | 18 | 16 (88.9) | 12 | 7 (58.3) | 97 | 77 (79.4) |
| Native Americans | 9 | 7 (77.8) | 48 | 38 (79.2) | 23 | 17 (73.9) |
| Asian | 4 | 3 (75.0) | 9 | 9 (100.0) | 22 | 16 (72.7) |
| Family history of COPD, bronchitis or emphysema | P=.158 | P=.145 | P=.559 | |||
| No | 797 | 678 (85.1) | 978 | 821 (84.0) | 821 | 613 (74.7) |
| Yes | 86 | 78 (90.7) | 193 | 170 (88.1) | 141 | 102 (72.3) |
| Schooling level (years) | P=.425 | P=.854 | P=.022 | |||
| 0–2 | 51 | 45 (88.2) | 78 | 66 (84.6) | 222 | 178 (80.2) |
| 3–4 | 136 | 116 (85.3) | 117 | 98 (83.8) | 294 | 225 (76.5) |
| 5–8 | 345 | 288 (83.5) | 351 | 293 (83.5) | 222 | 153 (68.9) |
| ≥9 | 349 | 306 (87.7) | 627 | 536 (85.5) | 222 | 157 (70.7) |
| Smoking status | P=.560 | P=.005 | P=.001 | |||
| Never smoked | 373 | 317 (85.0) | 384 | 340 (88.5) | 409 | 292 (71.4) |
| Ex-smoker | 258 | 226 (87.6) | 335 | 287 (85.7) | 316 | 259 (82.0) |
| Current smoker | 253 | 214 (84.6) | 454 | 366 (80.6) | 237 | 164 (69.2) |
| Lifetime cigarettes smoked | P=.127 | P=.023 | P<.001 | |||
| Never smoked | 373 | 317 (85.0) | 387 | 343 (88.6) | 409 | 292 (71.4) |
| ≤1 pack-year | 27 | 26 (96.3) | 80 | 68 (85.0) | 154 | 130 (84.4) |
| 1.1–10 pack-years | 116 | 105 (90.5) | 325 | 261 (80.3) | 199 | 159 (79.9) |
| >10 pack-years | 368 | 309 (84.0) | 379 | 319 (84.2) | 190 | 127 (66.8) |
| Passive smoking | P=.803 | P=.304 | P=.499 | |||
| No | 607 | 521 (85.8) | 673 | 576 (85.6) | 673 | 496 (73.7) |
| Yes | 277 | 236 (85.2) | 500 | 417 (83.4) | 289 | 219 (75.8) |
| Waist circumference (cm) (mean [SD]) | P=.256 | P=.482 | P=.635 | |||
| Below cut-off | 467 | 394 (84.4) | 684 | 575 (84.1) | 531 | 398 (75.0) |
| Above cut-off (≥88 for females or ≥102 for males) | 417 | 363 (87.1) | 485 | 415 (85.6) | 432 | 318 (73.6) |
| Body mass index (kg/m2) | P=.122 | P=.427 | P=.216 | |||
| <18.5 | 15 | 15 (100.0) | 6 | 5 (83.3) | 20 | 17 (85.0) |
| 18.5–24.9 | 242 | 199 (82.2) | 288 | 237 (82.3) | 342 | 257 (75.2) |
| 25–29.9 | 327 | 280 (85.6) | 499 | 421 (84.4) | 359 | 255 (71.0) |
| ≥30 | 300 | 263 (87.7) | 375 | 326 (86.9) | 242 | 187 (77.3) |
The highest missing numbers were 9 for skin color in Uruguay, 5 for BMI in Chile and 11 for pack-years in Brazil.
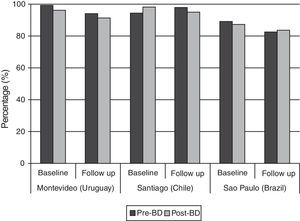
Regarding the overall quality of spirometries in both phases of the study, 80% or more of the tests achieved the ATS criteria in all centers (Fig. 1); it can also be observed that baseline spirometries showed a higher quality than follow-up tests (Fig. 1).
The death certificates were obtained from the national death registry of each country, with 76.1% of the death certificates being located in Montevideo, 88.3% in Santiago and 84.5% in Sao Paulo.
DiscussionTo the best of our knowledge, the PLATINO study is the first longitudinal population-based investigation addressing COPD in Latin America and will likely provide important information on the natural history of the disease. As its sample is representative of the adult population over 40 years of age in urban areas of Latin America, it can also contribute to the evaluation of other outcomes, such as overall mortality, the impact of public health policies (particularly on smoking), comorbidities (heart disease, hypertension, diabetes and lung cancer) as well as the management of patients with COPD.
The literature offers a number of important, large studies on the natural history of COPD and associated mortality rates. However, most of these investigations are not population-based studies.24–26 In Spain, there are two cross-sectional population based-studies (IBERPOC and EPI-SCAN) aimed at comparing the trends on the prevalence of COPD.27 The authors found a decline of 50.4% in the prevalence of COPD over a 10-year period. The results of these two studies are very relevant, but they cannot be analyzed as a cohort design since they were carried out in two different populations.
The fact that we could work with the same principal investigators in the three centers and some of the technicians from the baseline study helped ensure the quality of the data. However, some limitations of the study should be mentioned, such as the high cost of follow-up studies, problems involved in taking blood samples at home and the adequacy of vital statistics in some countries of Latin America. These points are addressed below.
Since funding was obtained gradually, each of the three centers had different follow-up periods. Thus, all future analyses must take these intervals into account and censored data will be used for the analysis of mortality.28 Such strategies should minimize the potential bias caused by follow-up visits with different intervals.
Blood samples collected at home require a large amount of work and high cost. For safety reasons, the decision was made to work with technicians from well-known clinical laboratories at each center. Due to the need for fasting and delays among the laboratory staff in reaching the homes, subjects who had agreed to give blood samples sometimes became refusals. Although detailed information on the address of the households was provided, it was not always easy to locate the correct home. Moreover, due to time restraints between blood collection and processing at the laboratory (a mean of 3h at all centers), few subjects were scheduled for each day. The main complaint of the subjects (especially elderly individuals) regarded the fasting period. Despite these difficulties, the overall blood sample rate was reasonably high (around 70%). The same protocol for collecting and processing the samples was followed rigorously at all centers.29,30
Some limitations in this study should be pointed out. One of them is the evaluation of trends or the decline of lung function. We have two cross sectional studies over time carried out with the same population; therefore, the design of the study is a cohort. However, for some outcomes such as natural history of a disease or reduction of lung function over time, two points are not enough for the evaluation of temporal trends. On the other hand, they can establish a pattern of the outcomes under study. It is our intention to have a third follow-up of the PLATINO study in the future.
Another point that can be considered a limitation of the study is the dose of bronchodilator (200mcg) administered to the subjects before the second spirometry; although the new GOLD guidelines propose a dose of 400mcg we thought it would be better for comparison with the PLATINO baseline study to have the same bronchodilator doses; otherwise, any future result could be attributable to the increased doses of bronchodilator.
Mortality data were the most important limitation of the present study. This information was extracted from official death certificates, which posed some problems. It was not possible to find certificates for some of the subjects who, according to the families, had died (23.9% in Uruguay, 11.7% in Chile and 8.2% in Brazil). Moreover, a considerable number of the death certificates were not properly filled out by the physicians, mainly in Uruguay. To improve the quality of the mortality data, the decision was made to have an expert on mortality records to recode the death certificates and certify the basic cause of death.31,32 This expert was blind to whether the subjects having died were diagnosed with COPD or not. The quality of the information on the death certificates from Santiago and Sao Paulo was considered as very adequate by the expert.
Despite these limitations, we encourage researchers in Latin America to conduct similar studies. It is our duty to show health authorities that vital statistics must be improved in Latin America to allow comparisons with data from other regions. Moreover, the creation of central laboratories, such as biobanks, would optimize time, costs and safety in the storage of biological material. Surveys of this type strengthen capacity building in Latin America and joint efforts allow higher standards of scientific knowledge to be achieved.
Authors’ ContributionsA.M.B. Menezes was the general coordinator of the PLATINO study, wrote the draft and reviewed the final version of this paper. M.M. de Oca also reviewed the manuscript. A. Muiño, M.V. Lopez-Varela, G. Valdivia, C. Lisboa and J.R. Jardim were the local coordinators of the PLATINO study. R. Perez-Padilla was responsible for spirometry quality control. F.C. Wehrmeister performed the statistical analysis. All authors read and approved the final version of manuscript.
FundingThe study was funded by the Associación Latinoamericana de Tórax (ALAT), Boehringer Ingelheim Pharma GmbH & Co. KG, GlaxoSmithKline and Novartis.
Conflict of InterestsThe authors declare no conflict of interests regarding this paper.
Members PLATINUM study team (in addition to lead authors) Maria Marquez, Maria White, Fernanda Rosa, Achilles Camelier, Francisco Franco, Dolores Moreno and Julio Pertuzé.
Team members are listed in Appendix 1.
Please cite this article as: Menezes AMB, Muiño A, López-Varela MV, Valdivia G, Lisboa C, Jardim JR, et al. Estudio de cohorte de base poblacional sobre la enfermedad pulmonar obstructiva crónica en Latinoamérica: métodos y resultados preliminares. Fase II del estudio PLATINO. Arch Bronconeumol. 2014;50:10–17.