The aim of this review is to give an overview of the clinical circumstances presenting before lung transplant that may have negative repercussions on the long and short-term prognosis of the transplant. Methods for screening and diagnosis of common comorbidities with negative impact on the prognosis of the transplant are proposed, both for pulmonary and extrapulmonary diseases, and measures aimed at correcting these factors are discussed. Coordination and information exchange between referral centers and transplant centers would allow these comorbidities to be detected and corrected, with the aim of minimizing the risks and improving the life expectancy of transplant receivers.
Esta revisión pretende exponer de forma sucinta aquellas circunstancias clínicas previas al trasplante pulmonar que pueden repercutir negativamente en el pronóstico del trasplante a corto y largo plazo. Se plantean los métodos de rastreo y diagnóstico de comorbilidades comunes de impacto pronóstico negativo sobre el trasplante, tanto de patologías pulmonares como extrapulmonares, y se proponen medidas dirigidas a su corrección. La coordinación y el intercambio de información entre los centros que remiten a los candidatos y los centros trasplantadores permitirán detectar y corregir estas comorbilidades con el fin de minimizar los riesgos y de mejorar las expectativas de supervivencia de los pacientes trasplantados.
Lung transplantation (LT) has taken its place as a therapeutic option for patients with advanced respiratory disease when all other available treatments are exhausted. LT results in terms of improved quality of life and life expectancy have increased demand in transplant units.
Transplant teams have accepted the clinical circumstances agreed to be absolute contraindications by consensus of experts.1,2 However, there are differences in our approach and in the risks we are willing to take in patients with various comorbidities that add up to a negative prognostic impact after transplantation.
The aim of this article is provide a succinct definition of those comorbidities that may impact on the transplant process and which can be detected, and possibly corrected, before the candidate is referred to the transplant unit or included on the waiting list. The ultimate goal would be to minimize the risks related to the procedure, and to improve transplant outcomes in terms of survival and quality of life. The authors believe that the best methodology for achieving this goal is to set up specialized units with multidisciplinary monitoring of these patients, similar to the care provided to cystic fibrosis patients. These units should collaborate with the transplant teams, creating protocols for detection and management of comorbidities.
Detection and Management of ComorbiditiesFor educational purposes, we have classified comorbidities as pulmonary and extrapulmonary. However, this order is not intended to imply a degree of prevalence or prognostic relevance. These aspects will be clearly defined below.
Pulmonary ComorbiditiesControl of Infection Before TransplantInfections are one of the most significant causes of death in transplant patients. In the perioperative period, infections share the limelight with primary graft dysfunction and with chronic rejection at later stages.3 They can be transmitted from the donor, acquired de novo after transplantation, be a reactivation of a latent infection in the host, or the proliferation of pathogens previously identified in patients with chronic bronchial infection/colonization before transplant. The first two points go beyond the scope this review.
In order to prevent reactivation of latent infections, the implementation of appropriate detection procedures is crucial. These include the assessment of the serological response to cytomegalovirus (CMV), Epstein–Barr virus (EBV), hepatitis virus A, B and C, human immunodeficiency virus (HIV), and the Mantoux skin test. Seropositive results for CMV and EBV before referral of the candidate will not define any particular attitude. However, this is essential knowledge for transplant teams. With this information, the risk of CMV disease after transplantation or EBV-associated lymphomas will be assessed, and appropriate preventive and monitoring measures will be implemented.
Active pulmonary tuberculosis (TB) is a contraindication for LT.1,2 Although TB may occur as a de novo infection after transplantation or be transmitted by the donor organ, TB after transplantation is explained in most cases by the reactivation of a latent infection in the recipient. LT is the solid organ transplantation with the highest risk of developing active TB.4 Moreover, mortality directly attributable to TB after lung transplantation is not negligible, and is mainly associated with a delay in diagnosis due to an atypical presentation or lack of completion of the appropriate treatment (drug toxicity, interaction with immunosuppressants, etc.).4,5 Detection and treatment of latent infection are therefore essential, and the Mantoux skin test should be carried out in LT candidates. Patients with positive Mantoux to be included on the waiting list should be appropriately treated, following the criteria of the Spanish consensus document.6 In order to shorten treatment periods, the common practice in our group is accelerated prophylaxis for 3 months with isoniazid and rifampicin (3RH).
In most cases, the microorganisms identified in early infections after LT are often the same pathogens that colonized the airway prior to LT. Therefore, it is essential that their impact is minimized, with culture negativization or, at least, control of chronic bronchial suppuration before LT. Whatever the underlying disease, the most relevant pathogens from the prognostic point of view are Pseudomonas aeruginosa, other multidrug-resistant (MDR) pathogens (Burkholderia cepacia, Achromobacter xylosoxidans, Stenotrophomonas maltophilia and methicillin-resistant Staphylococcus aureus), Aspergillus spp and non-tuberculous mycobacteria.
Pseudomonas aeruginosa (PsA) is the most frequently isolated colonizing microorganism in patients with cystic fibrosis (CF)7 and, given the continued antibiotic pressure (systemic or nebulized), and its hypermutability, identification of MDR strains is frequent. Pretransplant PsA colonization, especially with MDR strains, is associated with a rapid decline in lung function, accelerating the need for LT.8 The objective is to reduce bacterial load before LT, optimizing chest physiotherapy, with medical treatment to promote secretion drainage, and specific preparations of nebulized antibiotics (tobramycin, sodium colistimethate, or aztreonam). After transplantation, patients colonized with MDR PsA have decreased survival compared with those colonized by non-MDR strains.9
There is no evidence on the prognostic impact of other emerging multiresistant pathogens in LT. In CF units, the isolation rate of methicillin-resistant Staphylococcus aureus (MRSA) is increasing exponentially.7 Its presence prior to LT is associated with a worse prognosis10 and increased mortality in the first three months after transplant, due to increased infections of the lower respiratory tract with secondary bacteremia.11 The goal is again to decrease the bacterial load. To this end, the pharmacologic and non-pharmacologic measures defined above will be implemented, although no specific antibiotics for nebulized use are available at present. In clinical practice, tolerance to nebulized vancomycin can be assessed in patients with MRSA isolates and poor outcome.
The Burkholderia cepacia complex includes 17 bacterial subspecies with varying degrees of virulence. While most genomovars of Burkholderia cepacia do not have a prognostic impact either before or after LT, repeated isolation of Burkholderia cenocepacia (BCc, genomovar III) is associated with a significant increase in mortality before and after LT, especially in the first year.12,13 Neither control of immunosuppression nor aggressive antibiotic therapy appear to modify, in general terms, the poor prognosis of transplantation in these patients. This has led several international groups to consider chronic BCc colonization an absolute contraindication for LT.12,13 The Spanish groups have considered this as a relative contraindication.2
Between 10% and 25% of CF patients present Aspergillus spp isolates, usually Aspergillus fumigatus, especially those with lower functional capacity, frequent hospitalizations and marked bronchiectasis on radiological explorations.14 However, it is unclear whether this plays a role in loss of lung function, and whether a specific therapeutic intervention is required.14,15 Of the solid organ transplants, LT has the highest incidence of invasive fungal infection, but the isolation of Aspergillus spp. may range from a simple colonization to an invading disease.16 As a rule, it is assumed that its presence before transplant does not predict occurrence after LT, nor does it worsen post-transplant prognosis.17 However, it appears to increase the incidence of Aspergillus tracheobronchitis and complications in bronchial anastomosis.18 Therefore, there are no uniformly accepted criteria on the need for specific treatment of repeated isolates of Aspergillus or the most suitable treatment. It stands to reason that all chronic bronchial colonizations should be treated equally, with an individual assessment of the advantages and disadvantages of systemic and/or inhaled antifungals. Other fungi such as Scedosporium spp are less prevalent, but show a high incidence of recurrence after lung transplantation.
The prevalence of non-tuberculous mycobacteria in CF will increase in line with the age of the patients and their functional impairment. Not all species have the same clinical behavior.19 Identification of Mycobacterium avium complex before transplantation does not appear to be associated with poor prognosis after the procedure. Mycobacterium abscessus is a very virulent pathogen with a high risk for recurrence and invasive disease after LT.20 Therefore, aggressive treatment and repeated negative cultures for 12 months are required before the patient is included in the waiting list.21,22
Sequelae of Pleuropulmonary Processes and Chest DeformitiesPrior chest surgery is not a contraindication for LT unless it causes serious deformities, but it may complicate the explant surgery, increasing the risk of bleeding and prolonging ischemic times. These circumstances may lead to hemodynamic deterioration and increased risk of primary graft dysfunction. If pneumothorax must be managed before LT, extensive pleurodesis and talc use should be avoided.23
The importance of prior TB in a LT candidate, other than the risk of tuberculosis reactivation, depends on the extent and severity of the pleuropulmonary consequences. As a general rule, pleural thickening or pleural adhesions are not a contraindication in themselves, except in the case of extensive pleural calcification. In these cases, there is an increased risk of hemorrhage during surgery with a difficult explant and re-intervention for bleeding. Significant asymmetry in lung size, due to TB sequelae, recurrent infections with lobar collapse or kyphoscoliosis, may hinder implantation into a small cavity (very posterior hila with a high degree of cardiac manipulation, retracted or small hila, calcified mediastinal lymphadenopathy, etc.).
Invasive Mechanical VentilationThe need for invasive mechanical ventilation (IMV) in a patient under consideration for transplant not previously known to the team is currently a contraindication to LT.2 IMV before LT is a clear risk factor for mortality after LT.3 In patients on the waiting list that require IMV due to disease progression or unexpected acute deterioration, the need and the risk of LT should be individually assessed. The accumulated experience of our team, analyzed in terms of early and late survival, suggests that only young patients generally with CF, free of sepsis, and in need of IMV due to disease progression are suitable LT candidates in these circumstances.24,25
Pulmonary Hypertension in Chronic Respiratory DiseasesThe true prevalence of pulmonary hypertension (PH) in end-stage respiratory diseases is unknown, since screening by echocardiography is not carried out in all patients. The literature indicates that the prevalence increases with disease severity, being generally mild (less than 30mmHg of mean pressure in pulmonary artery demonstrated by right heart catheterization), except in 1%–3% of patients with COPD, in whom an PH disproportionate to the severity of the spirometric obstruction or the hypoxemia in arterial gases may be observed.26,27 Among the pathophysiological mechanisms that account for PH, hypoxia is the most important, but the interrelated mechanisms of endothelial dysfunction, pulmonary vascular remodeling, inflammation, acidosis, parenchymal destruction, or dynamic hyperinflation, all of which are modulated by still unknown genetic factors, must not be forgotten.26,27 In any case, the presence of PH prior to transplantation is associated with poor prognosis and quality of life and increased frequency of exacerbations.26–28
In the various expert consensus documents, PH suggests that the patient should be put on the LT waiting list.1,2 PH does not seem to have negative prognostic impact on long-term survival after lung transplantation, but it does increase mortality on the waiting list and perioperative mortality.28–30 These patients tend to require exogenous cardiorespiratory support more frequently, both prior to and during the LT, with the resulting increase in associated complications.
Extrapulmonary ComorbiditiesNon-pulmonary InfectionsChronic sinusitis, present in virtually all patients with CF, has not been traditionally considered as a risk factor after lung transplantation. Some authors detected a correlation between nasal cavity sinus infection and the incidence of post-transplant tracheobronchitis and pneumonia. They also suggest the hypothesis that treatment of sinusitis could have a positive influence on the development of bronchiolitis obliterans syndrome (BOS) by reducing inflammation and bronchial colonization.31 This view is not universally shared. Other authors compared the prognosis in terms of survival and graft colonization between patients with and without prior sinus surgery before LT and found no significant differences.32 Lacking evidence, preventive surgery on sinuses cannot be universally indicated, but only in those patients in whom this is medically indicated.
In the current consensus documents, HIV screening and the presence of chronic liver disease caused by hepatitis B (HBV) and C (HCV) virus are considered an absolute contraindication for LT.1,2 Until recently, these patients were excluded due to the fear that a high level of immunosuppression, such as that needed in LT, would lead to chronic liver disease progressing to liver cirrhosis.33 Given the good control of HBV replication achieved with new treatments (lamivudine, adefovir and entecavir), indication for LT in patients with chronic inactive HBV liver disease can be individualized (positive surface antigen detection and absence of HBV replication following specific treatment).34 The most appropriate antiviral treatment before and after LT, its duration, the monitoring needed, the immunosuppression regimen in these patients and the expected drug interactions between antivirals and immunosuppressants are still to be defined.
The absence of a safe and effective antiviral treatment for the control of chronic HCV liver disease and the increased incidence of acute rejection after transplantation with the use of interferon as antiviral therapy35 means that, for the moment, this is maintained as an absolute contraindication for most LT groups.1,2
In HIV patients, combined antiretroviral therapy achieves control and stabilizes viral replication in most patients. The few successful solid organ transplant published in these patients36 should be balanced with the intensity of required immunosuppression, recommendations in consensus documents, organ availability and long-term outcomes, among other relevant aspects.
Cardiovascular ProblemsArterial hypertension is an independent risk factor for developing cardiovascular events such as coronary disease or other atheromatous disorders.37 Additionally, when other risk factors such as age, tobacco smoking, hypercholesterolemia or diabetes are present, their association has a multiplicative, rather than additive, effect. At present, hypertension does not entail a significant comorbidity for LT, but it must be closely controlled after transplant, as its prevalence and ensuing renal toxicity may be increased by the effect of immunosuppressants. In summary, arterial hypertension is a cardiovascular risk factor that has also been identified as a prognostic factor for the development of renal dysfunction after transplant.38,39
The concept of global cardiovascular risk refers to the probability of suffering an ischemic coronary event, ischemic cerebrovascular disease, or lower-limb ischemia. It must be assessed on an individual basis with detection of risk factors and predisposing factors, including classification of patients with risk scores (Framingham equation or color tables)37 in order to establish appropriate preventive measures. The ultimate goal is to minimize the incidence and severity of cardiovascular events after transplantation. At present, this disease accounts for 10% of the causes of early mortality in LT and more than 5% of late causes, with frequency increasing as patient survival increases.3
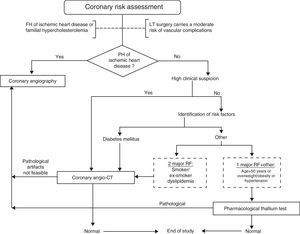
Nowadays, treatable and correctable ischemic heart disease (IHD) with preserved left ventricular function is no longer an absolute contraindication for LT.1,2 Its incidence among LT candidates is highly variable and, although respiratory diseases share many cardiovascular risk factors, they do not seem to predict significant IHD.40 LT is itself considered a process of moderate risk for cardiovascular complications, being a long surgical procedure with risk of poor oxygenation, hypovolemia and hypotension.41 Because perioperative mortality from cardiovascular events in LT is significant,3 as is the prevalence of silent and severe IHD, implementing a screening protocol for LT candidates seems reasonable. For whom and how are elements still to be defined. When only risk factors or their inclusion in the different scores for cardiovascular risk (Framingham or color tables) are taken into consideration, only between 60% and 65% of patients at risk are detected,42 so active case finding is needed. Although the gold standard for diagnosis is angiography, its invasive nature and the complications inherent to the procedure limit its generalization. Some non-invasive techniques are available, such as computed tomography (CT) coronary calcium scan, coronary CT angiography (CCTA) or magnetic resonance angiography for assessing anatomical changes, and others such as SPECT (single photon emission computed tomography), echocardiography or pharmacological thallium which assess myocardial function. These latter techniques are aimed more at assessing the damage caused by the IHD than at predicting the risk of future ischemic events. The difficulty lies in the integration of all the risk indices with non-invasive tests, primarily those that define coronary obstruction, in order to achieve a high negative predictive value while reducing invasive procedures.43 CCTA is emerging as the basic test for the diagnosis of coronary obstruction as a surrogate marker of clinically significant ischemic heart disease.44,45 However, besides the difficulties with contrast opacification or the presence of coronary calcifications, images may contain significant artifacts due to a lack of patient cooperation or the inability to achieve significant bradycardia in patients with advanced respiratory failure. Therefore, we consider the procedure followed by our LT group to be a practical screening proposal, as shown in Fig. 1. Other groups include a CT coronary calcium scan in their algorithms. In this examination, a score greater than 400 suggests a need for coronary angiography, while values between 100 and 400 should be further evaluated with coronary CT angiography.
A common problem is to convince cardiologists of the need to implement these measures in their screening, since absence of clinical symptoms does not rule out significant coronary obstruction. Similarly, a less significant coronary obstruction under standard conditions may have clinical implications, such as arrhythmias or angina caused by the stress to which the heart is subjected during LT. Therefore, active screening and aggressive treatment are required. We do not have any special recommendations for the potential LT population, other than the reperfusion techniques commonly implemented in interventional cardiology services, apart from the use of uncoated stents. These may entail an increased risk of late occlusion, but minimize the risk of suture dehiscence if LT is performed. Identification and treatment of coronary obstruction involves permanent antiplatelet therapy, associated with increased risk of bleeding during LT, but does not require specific treatment. If a stent is placed, a window of at least one month is recommended before inclusion on the waiting list, which is enough to discontinue the triple anticoagulation treatment and maintain permanent antiplatelet monotherapy.
Endocrine-metabolic ProblemsOsteoporosis is highly prevalent in patients on the waiting list for LT,46 appearing at a rate of over 50%. While this does not seem to have a direct impact on prognosis in terms of mortality, osteoporosis may affect progress after lung transplantation. In this period, osteoporosis increases the risk of fractures, and delays physical therapy and the incorporation of the patient to his/her daily activities as a result of pain. It also increases the risk of venous thromboembolism secondary to immobilization. Therefore, prediction of the fracture risk before LT is crucial, not only through bone densitometry but also with prognostic assessment scores47 that allow application of the most appropriate treatment for each clinical situation.
Nutritional status is an important prognostic predictor after LT. To this end, the Spanish consensus document for candidate selection includes an annex with a set of core data that should be collected in the clinical history.2 These include weight, height and body mass index (BMI) among others. Malnutrition or low body weight, defined as a BMI less than 17, have also been shown as a factor of poor prognosis,48,49 especially in patients with COPD and CF. In these, malnutrition increases mortality50 on the waitlist and in the first year after transplantation, especially in complex postoperative situations with prolonged stays in intensive care units, due to prolonged mechanical ventilation. Therefore, implementation of as many measures of nutritional support as deemed necessary to reverse the situation is important. Initial measures will be simple, such as adaptation of protein-calorie consumption to individual needs, followed by more complex measures, such as oral or enteral nutritional support or via a gastrostomy if continuous nocturnal feeding is necessary. Excess weight before transplant (BMI above 25kg/m2) is considered a predisposing factor for cardiovascular events,37 a perioperative risk factor for any surgery, and an independent risk factor for early mortality after LT, directly correlated with BMI increase.48,49 One study correlated obesity (BMI over 30) with the presence of severe acute graft dysfunction after LT.51 Like malnutrition, all necessary hygiene-diet and physical activity interventions should be undertaken in order to minimize risks before inclusion on the waiting list.
Immobilization or sedentary lifestyle is also considered a predisposing factor, although not a risk factor, of cardiovascular events. In addition, patients with extreme myopathy for whatever reason —lack of physical exercise, terminal respiratory failure, and use of corticosteroids or as a dimension of the multisystemic involvement of their underlying disease—have a poorer immediate prognosis, as determined by complications secondary to prolonged invasive mechanical ventilation after LT. Therefore, proper assessment by rehabilitation teams is necessary. If required, a training program for peripheral and respiratory muscles should be completed prior to LT in order to improve muscle strength and ventilatory mechanics.
Diabetes mellitus, besides being considered nowadays an ischemic equivalent and, therefore, of greater importance than the classical cardiovascular risk factors,37 is a risk factor for mortality before and after transplantation, and accelerates it being indicated in patients with CF.3,52,53 An Australian study associated diabetes before LT with a greater number of admissions for respiratory infections and acute rejection after LT.54 The small number of subjects included in the study precludes generalization of these findings.
Hyperlipidemias, which increase in frequency with age and the use of corticosteroids, are included as a cardiovascular risk factor when raised total cholesterol and LDL-cholesterol are identified, and as a conditioning factor in the case of hypertriglyceridemia. Therapeutic alternatives according to the risk level and the LDL-cholesterol target can be found in the Spanish document for secondary cardiovascular prevention.37,55
Chronic use of corticosteroids deserves special mention. These drugs are often and sometimes excessively used, although this is not supported by reliable studies and is even discouraged in consensus guidelines, such as the recommendations for stable COPD and idiopathic pulmonary fibrosis. Their use is not a comorbidity in itself, but the consequences are.17 These include osteoporosis, avascular necrosis of the hip, onset of diabetes or hypertension, and gastrointestinal and neuropsychiatric disease. International guidelines suggest that corticosteroid use does not contraindicate LT, but minimization of the dose administered is always recommended. The effect on mortality is probably conditioned by steroid myopathy, aggravated by the use of neuromuscular blocking agents during anesthesia, which negatively impacts on extubation, rehabilitation and discharge from intensive care units. During transplant surgery, the tissue and capillary fragility caused by chronic corticosteroid use promotes bleeding and renders vascular anastomosis difficult. It has also been proposed that secondary hyperglycemia may favor the development of severe infections.54 A safe minimum dose to ensure absence of risk cannot be defined. A retrospective study defined a cutoff of 0.42mg/kg/m2 per day for prednisone, above which postoperative survival was worse.56
Gastroesophageal RefluxGastroesophageal reflux disease (GERD) is defined as the presence of typical symptoms and/or complications in upper gastrointestinal endoscopy–esophagitis, ulcers, Barrett's esophagus, etc. –or histological confirmation of eosinophilic esophagitis in optically normal esophageal mucosa biopsies.57 This histological finding is characteristic but not pathognomonic of GERD. Its prevalence is highly variable, occurring in between 50% and 85% of patients with longstanding chronic respiratory diseases.58,59 Some authors dispute whether this association is causal or casual. This relationship has been studied in depth in CF, where it has been observed that reflux is secondary to the increased gastroesophageal pressure gradient, caused in turn by increased intrathoracic negative pressure during inspiration.60 That is to say, reflux is increased in disorders which require a large inspiratory effort, a condition common to the majority of patients listed for LT, with the exception of those with primary vascular disease.
The clinical features of GERD depend on the chemical composition of the reflux and its extent. While acid (gastroesophageal) reflux presents with classic symptoms (cough and bronchospasm by activation of vagal reflexes), bile (duodenogastroesophageal) reflux may occur without symptoms. The risk of proximal reflux, whatever its composition, is chronic microaspiration associated with pathogenic airway colonization,61,62 and the inflammatory or alloimmune response after LT. There is a growing number of publications linking GERD with bronchiolitis obliterans syndrome (BOS), which is equivalent to chronic rejection after LT.63–65 These are based on the identification of GERD as a trigger mechanism of chronic rejection, and the clinical evidence that effectively correcting reflux slows loss of pulmonary function,66 delaying the development and severity of BOS67,68 and improving survival of the graft and transplanted patients.67
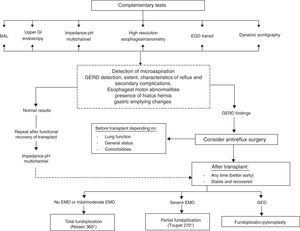
Clinical diagnosis is clearly insufficient. Neither the frequency nor the intensity of the symptoms are predictors of the degree of esophagitis, and their absence does not rule out GERD.57,69 Investigation of esophageal function and the presence of aspiration biomarkers (pepsin and bile acids) in bronchoalveolar lavage or histological evidence of aspiration in lung parenchyma is necessary.70–72 For a full study of the GERD spectrum, evaluation of the presence or absence of refluxed material, its bile or acid composition and extent (proximal or distal), including the presence of hiatus hernia or endoscopic abnormalities and esophageal motor activity, are all necessary. In reality, the technical complexity of the diagnostic procedures has meant that they are not widely implemented among transplant groups. Screening patients with only the more accessible techniques –manometry and pH measures for acid reflux detection –accounts for only part of the problem. These procedures may be relevant, but they are clearly insufficient since they do not detect silent bile reflux aspiration.
Pharmacological treatment of GERD with proton pump inhibitors is ineffective and even counterproductive. The therapeutic goal should be to prevent microaspiration and proton pump inhibitors (PPI) increase this. PPI increase non-acid reflux and the risk of bacterial colonization, as they buffer gastric pH.73–75 Therefore, only barrier or surgical methods are really effective. Patients with GERD and biomarkers or histological findings of aspiration will benefit from surgical correction.71,72,76 The time for carrying out this procedure, before or after LT, is defined by the degree of deterioration of lung function and the presence of comorbidities. In patients with respiratory failure, previous abdominal surgery, or pulmonary hypertension, pretransplant surgical correction is high risk. In any case, surgical intervention, whenever it is done, improves prognosis compared to patients with untreated GERD.67 Correction of GERD before transplantation, in addition to slowing the rate of functional impairment it produces in respiratory disease, provides immediate protection against BOS. A diagnostic-therapeutic algorithm that aims to give an overview of the complexity of GERD is shown in Fig. 2. Obviously each center will have to adapt this to their actual possibilities.
History of MalignancyThe risk of developing de novo malignancies in patients with solid organ transplants is very high compared to the general population,77,78 with the exception of breast and prostate cancer. Malignancies in transplants, de novo or due to recurrence in case of prior history, progress rapidly, have a worse prognosis and are more refractory to treatment.78 Therefore, it is essential to collect the transplant candidate's personal and family history of cancer. The record should contain histology and stage, treatment received and the disease-free period. In general, the risk of recurrence after transplantation is inversely proportional to the time free of malignant disease before the procedure.78 Expert consensus is quite lax in defining the time a patient should be disease-free before including the patient in the waiting list: the recommendations range from 2 to 5 years.1,2 Other more specific publications on this subject define better this period according to tumor type.78 For example, taking into account the most prevalent malignancies only, a window of more than three years for breast cancer is advised, five for invasive colorectal and cervical cancers, and two in the case of prostate cancer.
Diseases of Other Organ Transplants CombinedSome degree of chronic liver disease is frequently identified in CF patients before transplantation, particularly in the form of fibrosis, periportal inflammation or cholestasis. Even if histological liver cirrhosis with or without portal hypertension is common, this does not impact negatively on survival when LT is undertaken alone.79 By contrast, the association of liver disease with a severe nutritional disorder is a factor for poor prognosis.79 The need for combined lung–liver transplantation has to be considered in CF patients with liver cirrhosis and portal hypertension and uncontrollable complications (ascites, encephalopathy, etc.) or severe hepatic dysfunction.
At present, other simultaneous combined transplants, such as heart–lung and lung–kidney transplantation are exceptional. In patients with severe pulmonary hypertension with right ventricular dysfunction, perioperative support with extracorporeal membrane oxygenator will in most cases prevent the need for heart transplantation, with the exception of complex congenital heart disease. In patients with bronchiectasis and recurrent infections who have received multiple cycles of nephrotoxic antibiotics, the degree of proteinuria and renal dysfunction should be assessed for possible renal amyloidosis that may also require kidney transplantation.
Experiences of combined lung transplant with pancreatic islets80 or simultaneous pancreas-lung transplant81 in CF patients have been published. The aim is to cover much of the pathogenic spectrum of CF, such as exocrine and endocrine pancreatic insufficiency and respiratory failure. With simultaneous islet transplantation, insulin requirements were partially corrected but the deficits in the function of the exocrine pancreas remained. Lung-pancreas transplant from the same deceased donor might resolve this situation. However, the initial euphoria has been diminished by the availability of drug treatment for replacing pancreatic function, the increased surgical time, and the complications inherent to transplantation of each organ.
Psychological, Psychiatric or Psychosocial DisordersThe Spanish consensus on LT candidates2 includes three circumstances in this area that, if not properly corrected, constitute an absolute contraindication to transplantation: major psychiatric disorders, psychosocial or family breakdown that prevent specific care after transplantation, and repeated lack of adherence to treatment. Minor disorders in these fields require extensive care from the various departments involved for their correction before patients can be included on the waiting list for LT.
ConclusionOur final conclusion is that detection of comorbidities that impact on the post-lung transplantation prognosis of LT candidates and the implementation of measures to correct or control them (Table 1), is an appropriate approach for improving the prospects for survival transplant patients. Good coordination between transplant groups and referral centers, and a smooth flow of information between the various specialties involved, may certainly help improve the patient's condition before LT. The aim is to undertake surgery with as much guarantee as possible that full restoration of the patient's health and a long life expectancy can be achieved.
Pretransplant Comorbidities: Prognostic Impact, Detection and Proposals for Correction.
| Clinical situations | Impact on transplant | Detection method | Proposals for correction |
| Pulmonary comorbidities | |||
| Infection control before transplantation | |||
| Reactivation of latent infections | CMV dis. and EBV lymphoma | CMV IgG and M, EBV | Nothing particular |
| Tuberculosis infection | Risk of active TB with ↑ mortality | Mantoux | Accelerated prophylaxis 3RH |
| Control of chronic bronchial colonization | ↑ Early mortality, especially in MDR and recurrence | Sputum culture, fungi, and atypical mycobacteria | Specific treatment for ↓ bacterial load |
| Sequelae of pleuropulmonary processes and chest deformities | |||
| Previous thoracic surgery | ↑ ischemia time and bleeding risk | Imaging techniques | Avoid extensive pleurodesis and talc |
| Chest asymmetries | Difficult implant: greater manipulation | Imaging techniques | Nothing particular |
| Pretransplant mechanical ventilation | ↑ early mortality | – | Avoid as far as possible |
| Pulmonary hypertension | ↑ WL and perioperative mortality | Echocardiography, catheterization | Optimize treatment: O2, OSA, etc. |
| Extrapulmonary comorbidities | |||
| Non-pulmonary infections | |||
| Chronic sinusitis | ↑ Post-transplant infections? | CT of sinus en CF and bronchiectasis | Surgery if indicated |
| Chronic liver disease (HBV) | ↑ risk recurrence, progression to cirrhosis | HBV PCR | Negativization with antiviral drugs |
| Cardiovascular problems | |||
| Arterial hypertension | ↑ renal failure and cardiovascular dis. | Control of clinical and risk factors | Diet and drug treatment |
| Ischemic heart disease | ↑ perioperative mortality | Risk scales, coronary CT angiography, catheterization | Pretransplant repermeabilization techniques |
| Endocrine-metabolic problems | |||
| Osteoporosis | Pain, ↑ fracture risk and TED | Densitometry, FRAX index | Specific treatment before transplant |
| Malnutrition | ↑ mortality in WL and ↑ prolonged MV | BMI, laboratory results, endocrinology | Nutritional support |
| Obesity | ↑ early death↑ Early graft dysfunction? | BMI, endocrinology | Control. hygiene- diet + exercise |
| Immobilization/inactivity | ↑ risk of prolonged MV | Clinical history | Specific rehabilitation |
| Diabetes mellitus | ↑ Overall mortality and ↑ cardiovascular dis.↑ Infections and acute rejection? | History, laboratory, glycosylated hemoglobin, overload test | Diet and drug treatment |
| Hyperlipidemia | Risk of long-term cardiovascular dis.? | History, laboratory tests | Treatment according to risk and objectives |
| Chronic use of corticosteroids | ↑ Specific complications, ↑ mortality due to myopathy? Prolonged MV and capillary/tissue fragility? | Clinical history | Assess indication and minimize dose |
| Gastroesophageal reflux | Early and severe development of BOS and ↓ survival | BAL, manometry, pH monitoring, impedance, UGI, barium transit | Surgical correction before transplant if possible |
| History of malignancy | ↑ risk recurrence, early recurrence and poor prognosis | Family and personal history | Sufficient window |
| Diseases involving other organs | ↑ Transplant complexity | History and specific study | LT versus combined transplant |
| Psychosocial disorders | Early chronic rejection due to non-adherence | History, psychosocial study | Pharmacological treatment and psychosocial support |
Dis, disease; CMV, cytomegalovirus; EBV, Epstein–Barr virus; Ig, immunoglobulin; PT, pulmonary tuberculosis; RH, rifampicin+isoniazid; MDR, multi-drug resistant; WL, waitlist; CT; computed tomography; CF, cystic fibrosis; HBV, hepatitis B virus; TED, thromboembolic disease; MV, mechanical ventilation; BMI, body mass index; BOS, bronchiolitis obliterans syndrome; BAL, bronchoalveolar lavage; UGI, upper gastrointestinal endoscopy; LT, lung transplantation; OSA; obstructive sleep apnea syndrome; BMI, body mass index.
The authors declare no conflict of interest.
Please cite this article as: Vaquero Barrios JM, Redel Montero J, Santos Luna F. Comorbilidades con impacto pronóstico tras el trasplante pulmonar. Arch Bronconeumol. 2014;50:25–33.