To compare clinical outcomes and costs between two administration strategies of omalizumab treatment.
MethodWe evaluated two cohorts of patients with uncontrolled severe asthma over a 1-year period. Patients received the treatment in the primary care center in Hospital A and conventional hospital administration in Hospital B.
ResultsWe studied 130 patients, 86 in Hospital A and 44 in Hospital B, 30 men (24%) and 100 women (76%), age 50±15 years, FEV1% 67±22%, body mass index (BMI) 28±6kg/m2, 639±747UIIgE/mL, followed for 24±11 months (12–45), Asthma Control Test (ACT) score 12±4 and Asthma Control Questionnaire (ACQ) 3±2. There were no significant pretreatment differences between the groups in hospital admissions and emergency room visits in the previous year, nor in proportion of patients receiving oral steroids. Evaluations were performed at baseline and after 12 months of treatment, revealing significant differences in ACT (P<.001), ACQ (P<.001), improvement in FEV1% (P<.001), reduction in total admissions (P<.001), days of hospitalization (P<.001), emergency room visits (P<.001), cycles and doses of oral steroids (P<.001) compared to the previous year. Hospitalization costs, emergency room visits, unscheduled visits to primary care and to the pulmonologist were significantly reduced in each hospital and on the whole, but administration and travel costs were 35% lower in the ambulatory strategy adopted in Hospital A.
ConclusionThe administration of omalizumab in ambulatory health centers achieved the same clinical results as a hospital administration strategy, but with lower costs.
Evaluar los resultados clínicos y los costes de 2 estrategias de administración de omalizumab.
MétodoSe compararon, de forma retrospectiva, 2 cohortes de pacientes con asma grave no controlada: una, procedente del hospital A, en la que el tratamiento se administró en un centro de salud, y otra, procedente del Hospital B, con administración hospitalaria convencional.
ResultadosSe estudió a 130 pacientes, 86 en A y 44 en B, 30 hombres (24%) y 100 mujeres (76%), edad 50±15 años, FEV1% 67±22%, índice de masa corporal (IMC) 28±6kg/m2, IgE 639±747 UI/mL, seguimiento de 24±11 meses (12-45), Asthma Control Test (ACT) 12±4 y Asthma Control Questionnaire (ACQ) 3±2, sin diferencias significativas basales entre ambas cohortes en ingresos hospitalarios ni visitas a urgencias en el año previo, ni en número de pacientes con esteroides orales. Al comparar la situación basal y tras los 12 meses de tratamiento, se observaron diferencias significativas en ACT (p<0,001), ACQ (p<0,001) y mejoría en el FEV1% (p<0,001), reducción en número de ingresos (p<0,001), días de hospitalización (p<0,001), visitas a urgencias (p<0,001), ciclos y dosis de esteroides p<0,001) respecto al año previo, tanto individualmente como en conjunto. Los costes de hospitalización, visitas a urgencias, visitas no programadas a Primaria y al neumólogo se redujeron significativamente en ambos hospitales, pero los costes de administración y desplazamiento fueron un 35% inferiores con la pauta ambulatoria en A.
ConclusiónLa administración ambulatoria de omalizumab en los centros de salud consigue los mismos resultados clínicos que una pauta de administración hospitalaria, con menores costes.
Estimates suggest that in at least 5% of cases, asthma is severe and uncontrolled.1,2 The Spanish asthma management guidelines (GEMA) define severe uncontrolled asthma (SUA) as asthma that cannot be controlled despite administration of combined high-dose inhaled corticosteroids+long-acting beta-agonists (ICS+LABA) for 1 year, or oral corticosteroids for 6 months.3 Patients with a history of severe asthma have a 6-fold risk of a fatal outcome 3 years after hospital discharge. Furthermore, these patients have a higher risk of disease-related hospitalization, poor quality of life, high costs related to their disease, and mortality.1–4 Nevertheless, severe cases often remain undetected by both primary care and specialist physicians, because being, as they are usually are, confined to specialist asthma units,5 physicians working outside this context need not be skilled in their diagnosis.
Pharmacological asthma therapy has remained practically unchanged for many years. The first anti-IgE monoclonal antibody (omalizumab) has been available in Spain since 2006, and is indicated for SUA. Patients with severe asthma have a higher risk of morbidity and mortality, and absorb most of the asthma healthcare budget (70% of the total funds allocated to the Finnish asthma program, for example).6 Omalizumab has been shown to be effective in the management of severe asthma. Dose and dosing frequency of this drug is calculated from the patient's pretreatment IgE levels (IU/ml) and body weight (kg). Doses range from 75 to 600mg omalizumab every 15 or 30 days, adjusted to serum IgE.7–9
Although omalizumab can be administered in outpatient clinics, in Spain it is usually administered in a hospital, and the administration strategy is adapted to the specific setting, such as day clinics, pharmacy services, or pulmonology units. The method of administration and need for trained personnel add to the cost of the drug and increase the burden on the patient, who must bear the expense of traveling to the hospital to receive treatment. However, the effectiveness of outpatient vs. in-hospital administration, and the difference in cost between these strategies is as yet unknown.
The aim of this study was to compare the clinical outcomes and costs associated with omalizumab treatment in SUA patients in 2 tertiary hospitals. In one hospital, the drug was administered on an outpatient basis in a primary care center, and in the other it was administered in the day hospital.
MethodType of StudyRetrospective, population-based cohort study.
DurationTwelve months.
SettingAsthma units of the Pulmonology Departments of the Hospital Universitario San Juan in Alicante (hospital A) and the Consorcio Hospital General Universitario in Valencia (hospital B).
PopulationPatients with SUA included in an omalizumab treatment program in both centers, whose data was entered into a database, and who fulfilled the following criteria:
- –
Age>18 years.
- –
IgE>100IU/ml.
- –
Positive skin prick and/or positive specific IgE to airborne allergens.
- –
SUA criteria, treated with high-dose inhaled corticosteroids and a long-acting beta-agonist in combination with other drugs, such as oral leukotriene inhibitors and/or long-acting muscarinic antagonists and/or theophylline and/or steroids.3
- –
Uninterrupted administration of omalizumab for at least 12 months.
- –
To compare the economic cost of 2 different omalizumab administration strategies: day hospital and primary care center.
- –
To compare outcomes in both hospitals, in terms of exacerbations, hospitalization, lung function, and savings in medication following treatment with omalizumab for at least 12 months.
- –
To compare the number and characteristics of exacerbations, hospitalizations, lung function, and savings both before and after administration of omalizumab for at least 12 months in each hospital.
Strategy A. The drug was dispensed by the hospital pharmacy in a cooling pouch and collected by the patient. Both oral and written instructions were given for administration of the drug (including the first dose) in the nurse's treatment room in the patient's local primary care center.
Strategy B. The drug was dispensed by the hospital pharmacy and administered in the day clinic according to established protocols.
Study VariablesCost StudyHospital analytical accounting services estimated the cost of each of the following items:
- –
Hospital nursing costs.
- –
Primary care nursing costs.
- –
Average expenses incurred by the patient in traveling to the hospital or the primary care center.
- –
Cost of hospitalization/day.
- –
Cost of visit to emergency room.
- –
Cost of unscheduled visit to the primary healthcare physician.
- –
Cost of unscheduled visit to the specialist.
Each cost item was obtained and calculated specifically for each hospital service, according to the management guidelines laid down by the regional government (Generalitat Valenciana) for 2014.
Table 1 shows the cost allocated to each study variable in hospital A and B.
Analytical Costs of Each Study Variable Per Hospital (€).
| Analytical Costs | Hospital A | Hospital B |
|---|---|---|
| Non-surgical stay | 250 | 208 |
| Emergency treatment | 189 | 189 |
| First consultation with pulmonologist | 65 | 70 |
| Follow-up consultation with pulmonologist | 40 | 40 |
| First consultation scheduled with primary care physician | 57 | 57 |
| Follow-up scheduled consultation with primary care physician | 29 | 29 |
| Administration of drug by a nurse | 6 | 11 |
| Patient travel expenses | 3 | 6 |
- –
The clinical variables were: age, sex, comorbidity, treatment, atopy, dose administered, lung function, IgE, exacerbations, visits to the emergency room and hospitalization before and after indication for treatment, degree of asthma control measured using the validated Spanish versions of the Asthma Control Test (AST)10 and the Asthma Control Questionnaire (ACQ).11
- –
Use of asthma medication (long-term controller and quick relief) before and after omalizumab therapy.
Data from patients from both hospitals were entered into a single database and analyzed statistically using SPSS.
Numerical variables were analyzed descriptively (mean±SD). The Kolmogorov–Smirnov test was used to evaluate normal distribution. Following this, parametric (Student's t) and non-parametric (McWhitney) tests were performed to compare numerical variables from hospital A and B. Unpaired samples and also parametric and non-parametric (paired samples) tests were used to compare pre- and post-treatment clinical variables in patients receiving strategies A and B, and both groups together.
The chi-square test or Fisher's test were used to associate qualitative variables. In all tests, significance was set at P<.05.
Ethical MattersThe study was approved by the clinical trials ethics committee of the Consorcio H. General Universitario of Valencia, and assigned number CHI-OMA-2013-01. It was classified by the Spanish Ministry of Health, Consumer Affairs and Equality as an EPA-OD (post authorization study with a non-prospective follow-up), and being a retrospective study no informed consent from patients was required, although patient details included in the database were encrypted. The study adhered to the guidelines of the Declaration of Helsinki and the revised (Edinburgh 2000) version.
ResultsA total of 138 patients were initially included. Five from hospital A and 3 from hospital B were later excluded for the following reasons: lost to follow-up (3 cases), incomplete follow-up (less than 12 months, 3 cases), and dropout due to patient-perceived lack of improvement in 2 cases. The dropout rate (5.3% vs. 6.4%, P=ns) and the baseline characteristics of patients lost-to-follow-up were similar in both hospitals. The final sample included 130 patients, 86 from hospital A and 44 from hospital B.
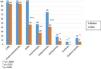
Table 2 shows the general characteristics of the patient population, together with comorbidities, smoking history, and treatment prior to the start of the study. Four patients from hospital A were diagnosed with allergic bronchopulmonary aspergillosis. Comorbidities and history of atopy were comparable in patients from both hospitals. All patients used short-acting bronchodilators on demand for quick relief; over 75% of patients used more than 4 puffs per week. One patient who was receiving daily oral steroids in combination with anticholinergics and antileukotrienes refused treatment with inhaled corticosteroids.
General Characteristics of Study Patients, Comorbidities, Smoking Habit and Background Therapy.
| Patients | 130 |
|---|---|
| Men | 30 (24%) |
| Women | 100 (76%) |
| Age (years, mean±SD) | 50±15years |
| FEV1% (mean±SD) | 67±22 |
| BMI (mean±SD) | 28±6kg/m2 |
| Follow-up (mean±SD) | 24±11 months |
| IgE (mean±SD) | 639±747IU/mL |
| ACT (mean±SD) | 12±4 |
| ACQ (mean±SD) | 3±2 |
| Comorbidity | |
| Obesity (BMI>30kg/m2) | 10% |
| Sleep apnea syndrome | 8.5% |
| Ischemic heart disease | 2% |
| Other pathology | 4% |
| Allergic rhinitis | 47% |
| Atopic dermatitis | 16% |
| Nasal polyps | 15% |
| Smoking habit | |
| Active smoker | 17% |
| Former smoker | 39% |
| Treatment | |
| Inhaled steroids | 99% |
| LABA | 99% |
| Antileukotrienes | 76% |
| LAMA | 48% |
| Antihistamines | 19% |
| Xanthines | 7% |
| Daily oral steroids | 16% |
ACT, Asthma Control Test score; ACQ, Asthma Control Questionnaire score; BMI, body mass index; FEV1%, forced expiratory volume in 1 second; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonists; SD, standard deviation.
A comparison of general characteristics of patients in the A vs. the B group before starting omalizumab showed no significant differences in age, sex, FEV1%, BMI, ACT and ACQ score, IgE levels, dose of inhaled corticosteroids, oral or controller steroid cycles, number of hospitalizations and number of visits to the emergency room over the preceding year. However, the aggregate number of days of hospitalization was greater in group A (P<.05) (Table 3).
Comparison of Pre-Treatment Clinical Characteristics by Hospital Group.
| Pretreatment | Hospital A | Hospital B | P |
|---|---|---|---|
| n | 86 | 44 | |
| Age (years, mean±SD) | 48±15 | 53±13 | n.s. |
| BMI (mean±SD) | 28±6 | 28±5 | n.s. |
| FEV1 (mean±SD) | 68±22 | 65±23 | n.s. |
| ACT (mean±SD) | 13±4 | 11±5 | n.s. |
| ACQ (mean±SD) | 3±2 | 3±1 | n.s. |
| Maximum inhaled steroid dosea | 92% | 89% | n.s. |
| Systemic steroid cycle (mean±SD) | 3±3 | 2±2 | n.s. |
| N.° hospitalization preceding year (mean±SD) | 2±1 | 2±1 | n.s. |
| Visits to emergency room preceding year (mean±SD) | 2±2 | 4±4 | n.s. |
| Monthly dose of omalizumab (mean±SD) | 540±250 | 476±316 | n.s. |
| Days of hospital stay preceding year (mean±SD) | 5±2 | 2±2 | <.05 |
ACT: Asthma Control Test score; ACQ: Asthma Control Questionnaire score; BMI: body mass index; FEV1%: forced expiratory volume in 1 second; n.s.: no statistical significance; SD: standard deviation.
A comparison of the pre-treatment and post-follow-up (12 months omalizumab) status of both groups (separately and pooled) showed significant differences in ACT (P<.001), ACQ (P<.001) and improved FEV1% (P<.001). In both groups, the total number of admissions fell significantly (P<.001), as did days of hospital stay (P<.001), visits to the emergency room (P<.001) and number of oral steroid cycles (P<.001) with respect to the preceding year. No inter-group post-treatment differences were found in these variables (Table 4).
Comparison of Clinical Characteristics Before and After 1-Year Omalizumab Therapy by Hospital and Total Patient Population.
| Hospital A | Hospital B | Total Patient Population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | P | Pre | Post | P | Pre | Post | P | |
| ACT | 13±4 | 21±5 | <.001 | 11±5 | 21±4 | <.001 | 12±4 | 21±4 | <.001 |
| ACQ | 3±1 | 2±1 | <.001 | 3±1 | 2±1 | <.001 | 3±1 | 2±1 | <.001 |
| FEV1% | 68±22 | 73±21 | <.01 | 64±24 | 71±26 | <.05 | 67±22 | 73±22 | <.001 |
| Hospitalizations/patient | 2±1 | 1±1 | <.001 | 2±1 | 1±1 | <.01 | 3±1 | 1±1 | <.001 |
| Visits to emergency room/patient | 3±1 | 1±1 | <.001 | 4±5 | 1±1 | <.001 | 4±3 | 1±1 | <.001 |
| Extra Primary Care visits/patient | 4±2 | 2±1 | <.001 | 5±2 | 1±1 | <.001 | 3±2 | 1±1 | <.001 |
| Visits to pulmonologist/patient | 3±1 | 1±1 | <.001 | 4±1 | 2±1 | <.001 | 3±2 | 1±1 | <.001 |
| Oral steroid cycles/patient | 3±3 | 1±1 | <.001 | 3±2 | 2±1 | <.001 | 3±3 | 2±2 | <.001 |
ACT: Asthma Control Test score; ACQ: Asthma Control Questionnaire score; FEV1%: forced expiratory volume in 1s.
Expressed as mean±standard deviation.
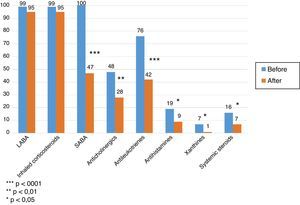
In both groups, the medication required for asthma control decreased significantly. The number of patients receiving inhaled corticosteroids remained the same, but in overall terms dose was reduced by 40% over pre-treatment levels. The proportion of patients using short-acting bronchodilators was significantly reduced (P<.001), as was use of antileukotrienes (P<.001), anticholinergics (P<.01), antihistamines (P<.05), and xanthines (P<.05). The proportion of patients requiring controller oral steroids was reduced (P<.05) (Fig. 1), together with their mean daily dose (22±12mg vs. 10±5mg, P<.01).
In terms of the safety profile of the study drug, only 10 patients presented initial flu-like syndrome, and 4 presented erythema and local induration, with no significant differences between cohorts. None of these adverse effects led to suspension of treatment or dropout.
In economic terms, costs associated with hospitalization, emergency consultations, unscheduled visits to primary care and the pulmonology department were significantly reduced in both hospitals (Table 5). Mean cost saving with respect to the preceding year (pre-treatment) was €1250 per patient/year.
Mean Cost Per Patient (€) Before and After 1-Year Omalizumab Therapy in Both Hospitals.
| Pre-Treatment | Post-Treatment | P | |
|---|---|---|---|
| Cost emergency visits | 484±737€ | 87±238€ | <.001 |
| Cost of hospitalization | 1022±1839€ | 402±1398€ | <.001 |
| Cost of pulmonologist consultation | 201±59€ | 152±39€ | <.001 |
| Cost of Primary Care consultation | 172±67€ | 59±30€ | <.001 |
Data expressed as mean±standard deviation.
Table 6 compares costs in group A and B, according to omalizumab administration strategy. Costs stemming from drug administration, nursing and patient travel expenses were lower in group A. No significant inter-group differences were found in other itemized costs, with the exception of travel expenses associated with visits to primary care and the pulmonology department before start of omalizumab treatment, which were lower in group A (Table 6). Mean annual savings per patient in nursing and travel to and from the day clinic or primary care was €100 per patient/year, which in proportional terms is a reduction of 35% between the 2 treatment strategies.
Mean Analytical Costs Per Patient in Both Hospitals.
| Hospital A | Hospital B | P | |
|---|---|---|---|
| Pre-treatment emergency room cost | 398±438€ | 653±1098€ | n.s. |
| Post-treatment emergency room cost | 103±282€ | 56±104€ | n.s. |
| Pre-treatment hospitalization cost | 1308±2168€ | 463±600€ | <.05 |
| Post-treatment hospitalization cost | 552±1690€ | 109±289€ | n.s. |
| Pretreatment Primary Care cost | 160±62€ | 198±69€ | <.01 |
| Post-treatment Primary Care cost | 57±29€ | 64±31€ | n.s. |
| Pretreatment pulmonologist cost | 191±62€ | 220±49€ | <.01 |
| Post-treatment pulmonologist cost | 156±41€ | 145±34€ | n.s. |
| Nursing care cost | 182±50€ | 278±99€ | <.001 |
| Travel expenses | 61±17€ | 98±35€ | <.001 |
Data expressed as mean±standard deviation.
Although omalizumab has been shown to be effective in the treatment of SUA,12 to the best of our knowledge no studies have evaluated and compared the cost and clinical outcomes of in-hospital and outpatient administration of this therapy. Our findings have shown that in 2 comparable populations of patients with severe asthma, outpatient administration of the drug is perfectly feasible. Clinical outcomes in both populations were similar, but the cost of outpatient administration was lower for both the hospital and the patient, and more convenient for the latter. Some cost differences were attributable to the higher cost of stay in the hospital where the drug was administered on an outpatient basis, while the difference in costs derived from visits to the primary care physician and the specialist before starting treatment was less significant. The cost of hospital stay is inherent to the characteristics of each hospital, and depends on various factors such as infrastructure overheads, number of beds, maintenance costs, staff, etc.13 and the cost of the initial and subsequent visits will also depend on structural overheads, staff costs, or the cost of scheduled appointments. All these are intrinsic to hospital management and are usually specific to each hospital; thus, these costs are variable and unlikely to change in the short term.
Omalizumab should only be prescribed by specialists. In Spain, it is usually administered in a hospital setting, probably because it is only available through hospital pharmacies. Omalizumab must be administered by a trained nurse in a controlled setting, although the scarcity of adverse effects does not fully justify these precautions. There is no evidence that in-hospital administration of the drug is safer or more clinically effective than administration in other healthcare settings, such as primary care. In this study, all patients were recruited from a specialist asthma unit where the drug was prescribed, the dosage adjusted and patients followed up in the usual manner. Follow-up of SUA patients receiving omalizumab has not hitherto been evaluated in other centers, such as primary care. The treatment itself is costly, and reducing the cost of administration would benefit the healthcare system. Some authors have estimated the total number of asthmatics in Spain to be 857514 patients, with an overall cost of €1480 million.14 Treating uncontrolled asthma accounts for 70% of this expenditure, including all non-medical costs, and certain direct medical costs (hospitalization, visits to the emergency room, fatality). In our study, the mean direct pre- and post-treatment medical costs per patient (such as visits to the emergency room, hospitalization and non-scheduled visits to primary care and specialist physicians), despite considerable inter-individual variability, was reduced by €1200/year. If prevalence of SUA in the total asthmatic population in Spain is taken to be 5%, 45000 patients in Spain would be affected by the severe, uncontrolled form of this disease, most of whom are candidates for treatment with omalizumab or other biological therapies appearing in the future. Based on our findings, the cost of omalizumab can be reduced by 35% merely by modifying the administration strategy, giving an estimated saving of between €4.5 and €9 million/year.
As other authors have shown, omalizumab significantly reduces the number of hospitalizations for exacerbation, visits to the emergency room, non-scheduled visits to primary care and specialist physicians, and also the dose of inhaled and oral steroids.12,15–18 This was true of both our study groups, irrespective of whether the drug was administered in-hospital or in primary care. This finding supports the clinical benefit of omalizumab in SUA patients, and also suggests that administration in an outpatient setting is equally effective.
Several studies have analyzed omalizumab in terms of pharmoeconomics. Oba et al.19 estimated the cost of each additional successfully controlled day to be $523, and the daily cost of achieving at least a 0.5-point increase in Asthma Quality of Life Questionnaire score to be $378. The authors concluded that, from a pharmoeconomics perspective, omalizumab is more beneficial in SUA patients. They found this therapy to be cost-effective when used in non-smokers that are hospitalized 5 or more times each year, or for more than 20 days in total, despite receiving the best possible treatment.
Dewilde et al.20 estimated the cost-effectiveness of omalizumab to be €56091/quality of life year (QALY), assuming a fatal outcome in 1%–3% of exacerbations, and equating reduction in mortality with reduction of exacerbations. A recent cost-effectiveness study was able to establish a difference in favor of omalizumab in terms of chronic disease, exacerbation-related mortality, price, exacerbations and clinical response, and additional QALYs, even with increased direct medical costs. The authors concluded that the cost-effectiveness of omalizumab was similar to that of other chronic biological treatments.21
Another study showed that omalizumab progressively improved asthma control and quality of life while reducing the cost of medication and hospital treatment. Although costs increased by €450/month, cost effectiveness increased substantially by €23880 per QALY gained, showing the beneficial outcome of investing in omalizumab in developed countries.22 A study carried out in Spain reported an incremental cost-effectiveness ratio of €462.08 per prevented exacerbation, and 26864.89 per QALY gained.23 A recent review study has shown that current asthma treatment is cost-effective in terms of disease control and therapeutic choices, including inhaled steroids, long-acting bronchodilators, and omalizumab.24 It is important to bear in mind that cost-effectiveness measured by QALYs will differ according to the calculation method used, the chronicity and severity of the disease analyzed, and the reference healthcare system. This could explain the difference in results reported in different studies.25
Our study has some important limitations. It is a retrospective study performed in 2 asthma units specializing in patients with SUA. Nevertheless, although some bias may be present in sample selection, all patients completing a course of treatment lasting at least 1 year were included in the study, with very few exclusions. We believe that as our study was performed in a routine clinical practice setting, the results are conclusive. However, it might not be possible to infer results obtained from the group receiving the drug in an outpatient service to other units, due to differences in the geographical extent of the catchment population or the complexity of higher risk patients (severity, frequency, instability of the crisis, comorbidity). Factors such as the impact of the hospital on the local population, its location (central or distant), level of care, and organizational system used can cause major variations in cost.
In conclusion, our study has contributed valuable insights into the cost-effectiveness of omalizumab in SUA patients. We show that this drug can be administered in an outpatient setting, and that this strategy can help reduce the cost of this therapy. Outpatient administration of omalizumab did not compromise safety, achieved the same clinical outcomes, and was more convenient for patients.
FundingThis study was partially funded with a Novartis research grant awarded in 2014.
Authors’ ContributionECHV and EFB designed and planned the study, performed the statistical study, and wrote and revised the manuscript.
PL and LN compiled the database, evaluated the patients, and revised the manuscript.
MR, JNSCH, CS and JB evaluated study patients in their clinical practice, and helped draft and revise the manuscript.
Conflict of InterestsThe authors declare they have no conflicts of interest.
Please cite this article as: Chiner E, Fernández-Fabrellas E, Landete P, Novella L, Ramón M, Sancho-Chust JN, et al. Comparación de costes y resultados clínicos entre la administración hospitalaria o ambulatoria de omalizumab, en pacientes con asma grave no controlada. Arch Bronconeumol. 2016;52:211–216.