Trisegmentectomy, or resection of the upper subdivision of the left upper lobe with preservation of the lingula, is considered by some authors to be equivalent to right upper lobectomy with middle lobe preservation.
Our objective was to compare survival and recurrence after trisegmentectomy versus left upper lobectomy procedures registered in the Spanish Video-Assisted Thoracic Surgery group (GEVATS) database.
MethodsWe compared mortality, survival and recurrence in patients with left upper lobectomy or trisegmentectomy after propensity score matching for the following variables: age, smoking habit, tumor size, histologic type, radiological density of tumor, surgical access, forced expiratory volume in one second, diffusing capacity of the lungs for carbon monoxide, hypertension, chronic heart failure, ischemic heart disease, arrhythmia, stroke, peripheral vascular disease, diabetes and pre-surgery nodal status by positron emission tomography/computed tomography.
ResultsA total of 540 left upper lobectomies and 83 trisegmentectomies were registered in the GEVATS database. After propensity score matching, 134 left upper lobectomies and 67 trisegmentectomies were selected. Survival outcomes were similar, but differences were found for recurrence (21.5% for trisegmentectomies vs. 35.4% for left upper lobectomies, p=0.05). Moreover, the recurrence patterns differed, with the lobectomy group showing a greater tendency to distant dissemination.
ConclusionsTrisegmentectomy and left upper lobectomy show similar 5-year survival rates. In our database, recurrence after trisegmentectomy was lower than after left upper lobectomy, while the recurrence pattern differed among the 2 surgical approaches, with a greater tendency to distant metastasis after left upper lobectomy.
The extent of resections in lung tumors has been widely studied in recent years, and sublobar resection has been shown to have similar survival rates to lobectomy in peripheral lesions measuring less than 2cm with N0 status.1,2
Lingula-sparing trisegmentectomy is a multisegmentary technique that lies midway between segmentectomy and lobectomy. It is considered by some authors to be equivalent to right upper lobectomy with preservation of the middle lobe.3 Studies suggest that this surgical approach achieves good oncological outcomes beyond the limits established in clinical trials for conventional segmentectomies.4–9 Although evidence in this area is limited by the lack of studies, no differences in terms of survival were observed in 2 studies in disease stages IA and IB,5,6 although the patient numbers in stage IB were low.9
Our objective was to compare survival and recurrence after trisegmentectomy versus left upper lobectomy using propensity score techniques applied to the Spanish Video-Assisted Thoracic Surgery (GEVATS) database. Specifically, nearest neighbor propensity score matching methods were used. Complications between both types of resection were also compared.
Materials and MethodsDatabaseWe analyzed data from the Spanish Society of Thoracic Surgery GEVATS database, which recruited patients undergoing anatomic pulmonary resections between December 20, 2016 and March 20, 2018 who were followed up until July 31, 2022.10 This database prospectively included patients from 33 thoracic surgery units. The study was approved by the ethics committees of all participating centers. In this analysis, we included the 30 centers in which oncological follow-up for survival had been completed.
Of the 3533 patients included in the registry, patients with lung cancer (3085) were selected, and of these, individuals with a follow-up at least 90 days post-procedure (2726) were included in the study cohort. All left trisegmentectomies (anatomic resection S1–S3) and all left upper lobectomies in this group were selected, resulting in a total of 623 patients, comprising 540 lobectomies and 83 trisegmentectomies.
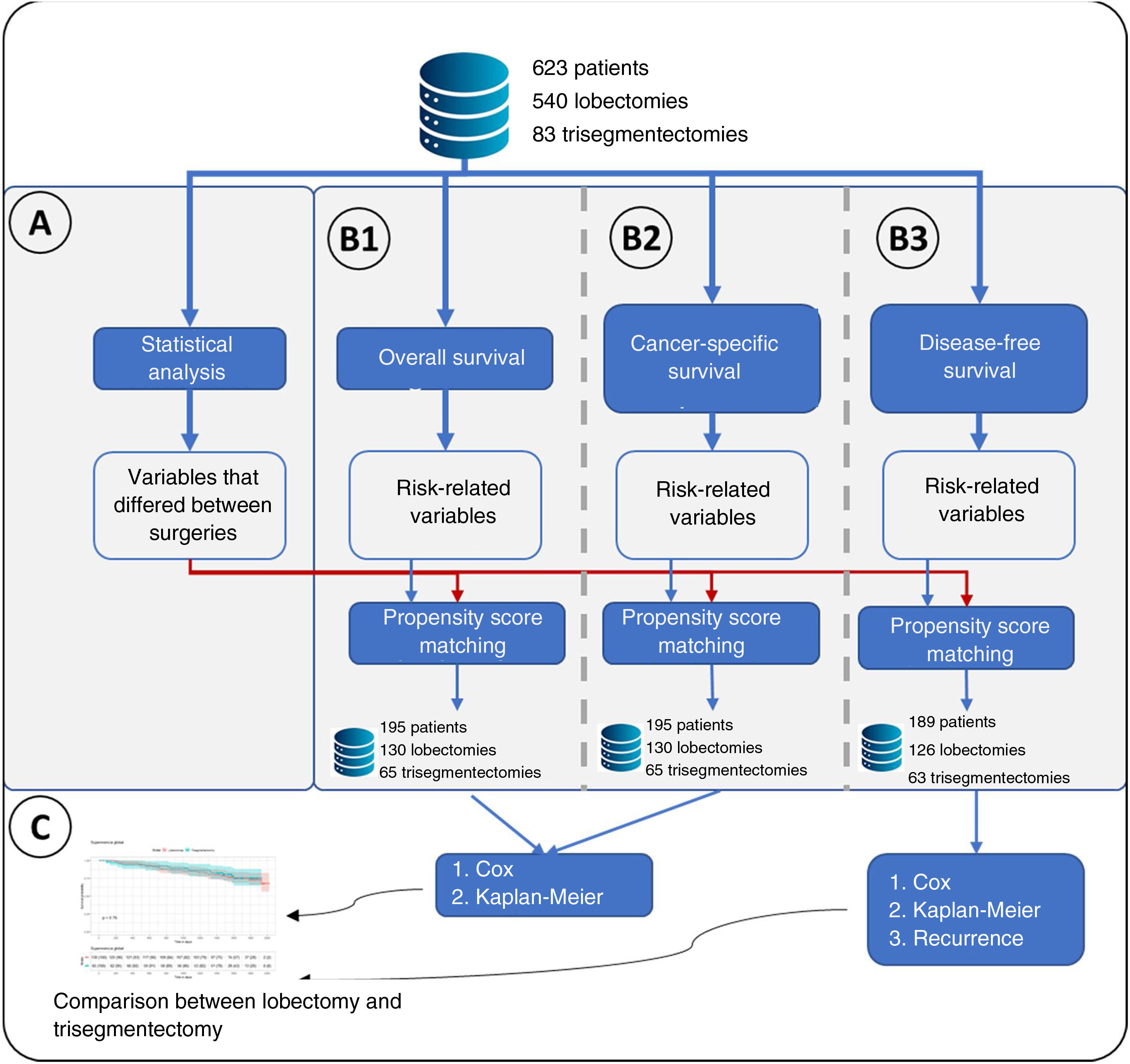
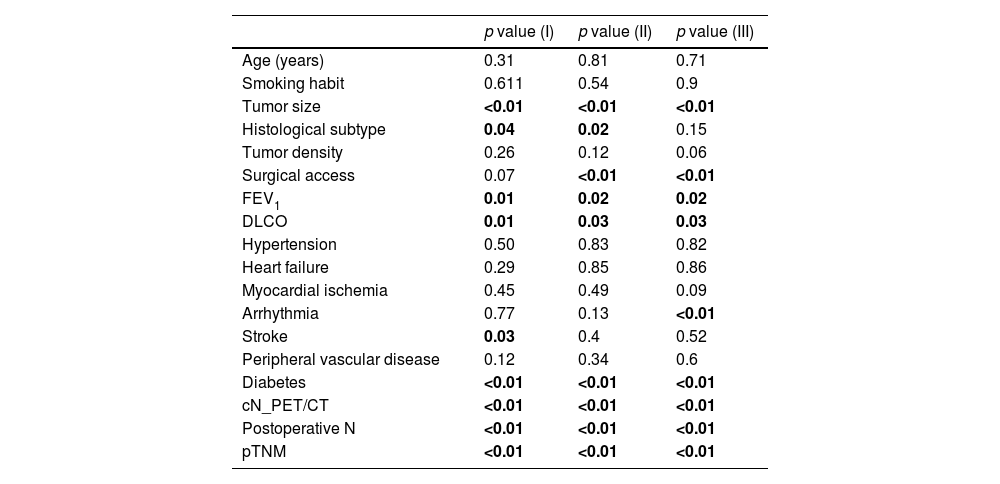
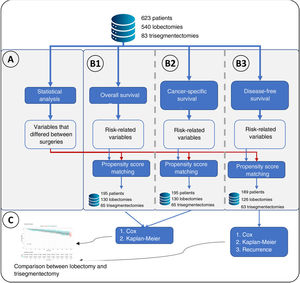
Statistical AnalysisThe following variables were compared for the 2 types of resection: age, smoking habit, tumor size, histological type, radiological density of the tumor, surgical access, forced expiratory volume in the first second (FEV1), diffusing capacity of the lungs for carbon monoxide (DLCO), hypertension, chronic heart failure, ischemic heart disease, arrhythmia, stroke, peripheral vascular disease, diabetes, cN status by positron emission tomography/computed tomography (PET/CT), pTNM, and postoperative N. Continuous variables were compared using a t-test, and categorical variables were compared using Fisher's exact test. A difference in any variable between both groups was considered significant if the p value was less than 0.05 (Fig. 1A).
Flowchart of the main methodology used. (A) Represents the statistical analysis performed comparing lobectomies and trisegmentectomies. This statistical analysis consisted of a t-test for continuous variables and Fisher's test for categorical variables. Statistical significance was set at a p value<0.05, obtaining a list of “variables that differed between surgeries”. (B) Represents the 3 types of survival analyses performed: B1: overall survival in which the event was death; B2: cancer-specific survival, in which the event was cancer-specific mortality; and B3: disease-free survival. A Cox regression was performed for the 3 analyses, and a variable was considered to have an effect if the p value of the Cox regression likelihood ratio test was <0.05. For the 3 analyses, propensity score matching was performed in which the “variables that differed between surgeries” plus the variables related to risk were considered. (C) Once the groups were obtained after propensity score matching, the effect of the type of surgery on the risk of overall survival, cancer-specific survival and disease-free survival was analyzed. Finally, for B3, the type of recurrence was analyzed using Fisher's test.
A survival analysis of each variable was performed using the Cox regression model. Specifically, 3 separate survival analyses were performed: (I) overall survival, in which the event being studied was all-cause mortality; (II) lung cancer-specific survival, in which the event being studied was cancer-specific mortality; and (III) disease-free survival, in which the event being studied was recurrence. A variable was considered to affect each of the analyses if the likelihood ratio test of the model had a p value less than 0.05 (Fig. 1B).
Creation of Comparable GroupsVariables that were significant in the statistical analysis or survival analysis were used to perform propensity score matching using the 2:1 nearest neighbor technique (Matchit library). Once comparable groups had been created, group homogeneity was verified using the t-test (continuous variables) and the Fisher's exact test (discrete variables) for the aforementioned variables (Fig. 1B).
Risk Comparison Between SurgeriesOnce the comparable groups had been created, overall survival, lung cancer-specific survival and disease-free survival were estimated using Kaplan–Meier curves, comparing both groups with the log-rank test. A Cox regression was also performed to compare the risk between both surgical approaches in all patients, using inverse probability of treatment weighting (IPTW) methodology.11 This method uses all patients, conferring to each one a weight according to the value obtained in the propensity score. These weights were calculated for each patient as 1/propensity score for patients with trisegmentectomy and 1/(1−propensity score) for the remaining patients, where propensity score is the score obtained.
Recurrence Type AnalysisFinally, recurrence and the recurrence pattern were compared using Fisher's test.
Subgroup Analysis of Tumors Measuring Between 20 and 30mmThe methodology described above and in Fig. 1 was applied to a subgroup of patients with tumor size between 20 and 30mm.
The statistical analysis was performed using R software and the Matchit library was used to calculate the propensity score.
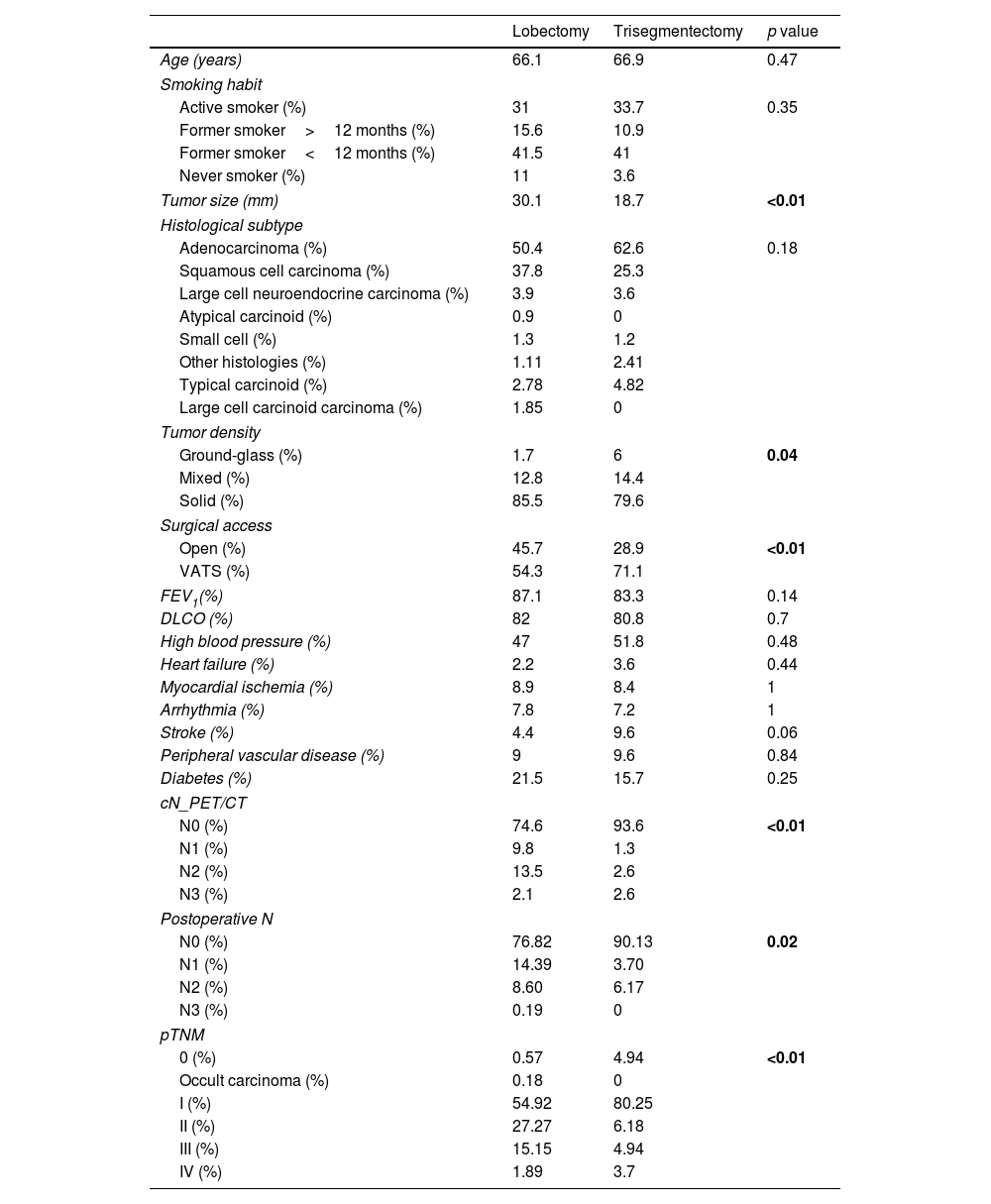
ResultsComplete Set AnalysisIn patients with a diagnosis of lung cancer and survival of more than 90 days, 540 left upper lobectomies and 83 trisegmentectomies were retrieved. Statistical analysis was performed to compare the variables of interest between the lobectomy and trisegmentectomy subgroups. The results of this analysis are summarized in Table 1. The variables associated with significant differences (p value<0.05) were: tumor size, tumor density, surgical access, cN, postoperative N and pTNM. Among these variables, tumor size is notable, with an average of 30mm in lobectomies and 18.7mm in trisegmentectomies. The differences in pTNM (p value=5.32e−08) between the 2 types of surgeries are also worth pointing out.
Baseline Characteristics of Both Groups (Lobectomies and Segmentectomies) Prior to Propensity Score Matching.
| Lobectomy | Trisegmentectomy | p value | |
|---|---|---|---|
| Age (years) | 66.1 | 66.9 | 0.47 |
| Smoking habit | |||
| Active smoker (%) | 31 | 33.7 | 0.35 |
| Former smoker>12 months (%) | 15.6 | 10.9 | |
| Former smoker<12 months (%) | 41.5 | 41 | |
| Never smoker (%) | 11 | 3.6 | |
| Tumor size (mm) | 30.1 | 18.7 | <0.01 |
| Histological subtype | |||
| Adenocarcinoma (%) | 50.4 | 62.6 | 0.18 |
| Squamous cell carcinoma (%) | 37.8 | 25.3 | |
| Large cell neuroendocrine carcinoma (%) | 3.9 | 3.6 | |
| Atypical carcinoid (%) | 0.9 | 0 | |
| Small cell (%) | 1.3 | 1.2 | |
| Other histologies (%) | 1.11 | 2.41 | |
| Typical carcinoid (%) | 2.78 | 4.82 | |
| Large cell carcinoid carcinoma (%) | 1.85 | 0 | |
| Tumor density | |||
| Ground-glass (%) | 1.7 | 6 | 0.04 |
| Mixed (%) | 12.8 | 14.4 | |
| Solid (%) | 85.5 | 79.6 | |
| Surgical access | |||
| Open (%) | 45.7 | 28.9 | <0.01 |
| VATS (%) | 54.3 | 71.1 | |
| FEV1(%) | 87.1 | 83.3 | 0.14 |
| DLCO (%) | 82 | 80.8 | 0.7 |
| High blood pressure (%) | 47 | 51.8 | 0.48 |
| Heart failure (%) | 2.2 | 3.6 | 0.44 |
| Myocardial ischemia (%) | 8.9 | 8.4 | 1 |
| Arrhythmia (%) | 7.8 | 7.2 | 1 |
| Stroke (%) | 4.4 | 9.6 | 0.06 |
| Peripheral vascular disease (%) | 9 | 9.6 | 0.84 |
| Diabetes (%) | 21.5 | 15.7 | 0.25 |
| cN_PET/CT | |||
| N0 (%) | 74.6 | 93.6 | <0.01 |
| N1 (%) | 9.8 | 1.3 | |
| N2 (%) | 13.5 | 2.6 | |
| N3 (%) | 2.1 | 2.6 | |
| Postoperative N | |||
| N0 (%) | 76.82 | 90.13 | 0.02 |
| N1 (%) | 14.39 | 3.70 | |
| N2 (%) | 8.60 | 6.17 | |
| N3 (%) | 0.19 | 0 | |
| pTNM | |||
| 0 (%) | 0.57 | 4.94 | <0.01 |
| Occult carcinoma (%) | 0.18 | 0 | |
| I (%) | 54.92 | 80.25 | |
| II (%) | 27.27 | 6.18 | |
| III (%) | 15.15 | 4.94 | |
| IV (%) | 1.89 | 3.7 | |
The p value was calculated from the t-test for continuous variables and the Fisher's exact test for categorical variables. The lobectomy and trisegmentectomy columns are presented as mean for continuous variables and percentage for categorical variables.
Table 2 shows the results for the 3 survival analyses (overall, cancer-specific, and disease-free survival). This table shows the p value of the Cox regression likelihood ratio test. See Supplementary Table 1 for the p value corresponding to each Cox regression coefficient.
Cox Regression Survival Analysis for Each Variable Selected Separately, Prior to Propensity Score Matching.
| p value (I) | p value (II) | p value (III) | |
|---|---|---|---|
| Age (years) | 0.31 | 0.81 | 0.71 |
| Smoking habit | 0.611 | 0.54 | 0.9 |
| Tumor size | <0.01 | <0.01 | <0.01 |
| Histological subtype | 0.04 | 0.02 | 0.15 |
| Tumor density | 0.26 | 0.12 | 0.06 |
| Surgical access | 0.07 | <0.01 | <0.01 |
| FEV1 | 0.01 | 0.02 | 0.02 |
| DLCO | 0.01 | 0.03 | 0.03 |
| Hypertension | 0.50 | 0.83 | 0.82 |
| Heart failure | 0.29 | 0.85 | 0.86 |
| Myocardial ischemia | 0.45 | 0.49 | 0.09 |
| Arrhythmia | 0.77 | 0.13 | <0.01 |
| Stroke | 0.03 | 0.4 | 0.52 |
| Peripheral vascular disease | 0.12 | 0.34 | 0.6 |
| Diabetes | <0.01 | <0.01 | <0.01 |
| cN_PET/CT | <0.01 | <0.01 | <0.01 |
| Postoperative N | <0.01 | <0.01 | <0.01 |
| pTNM | <0.01 | <0.01 | <0.01 |
The first column shows the p value of the overall survival analysis. The second column shows cancer-specific survival analysis and the last column shows disease-free survival. The p value is that of the Cox regression likelihood ratio test.
In the overall survival analysis, the following variables were significant (p value<0.05): tumor size, histological subtype, FEV1, DLCO, stroke, diabetes, cN, postoperative N, and pTNM.
In the analysis of cancer-specific survival, the following variables were significant: tumor size, histological subtype, surgical access, FEV1, DLCO, diabetes, cN, postoperative N and pTNM.
In the disease-free survival analysis, the following variables were significant: tumor size, histological subtype, surgical access, FEV1, DLCO, arrhythmia, diabetes, cN, postoperative N, and pTNM. In this analysis, 26 patients were excluded due to a lack of information on recurrence, leaving a total of 597 patients, with 517 lobectomies and 80 trisegmentectomies.
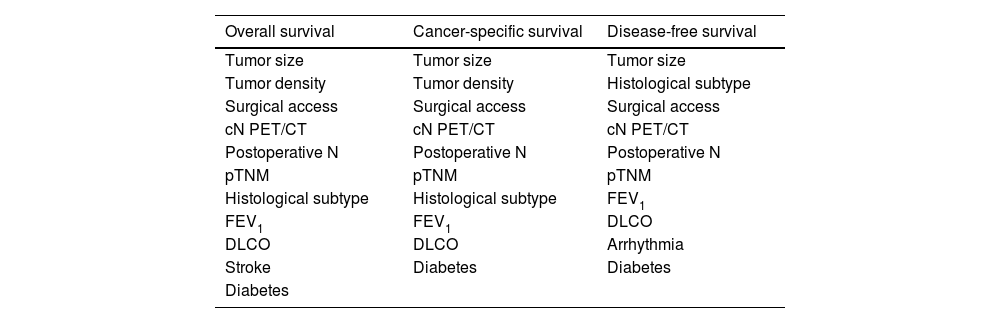
Creation of Comparable Groups Using the Complete SetPropensity score matching was performed with the variables that were significant in these analyses (Fig. 1B). In total, 3 types of matching were conducted, 1 for each type of analysis. To be able to use all variables, patients with incomplete information were excluded. In this way, a group of 195 patients with 130 left upper lobectomies and 65 trisegmentectomies was obtained for the first 2 analyses and 1 group of 189 patients was obtained for the third analysis, consisting of 126 lobectomies and 63 trisegmentectomies. Table 3 shows the variables used for each of the 3 analyses, the number of patients with complete and incomplete information, and finally the number of patients in each group after the propensity score procedure.
Variables Used for Propensity Score Matching for Each Type of Survival Analysis.
| Overall survival | Cancer-specific survival | Disease-free survival |
|---|---|---|
| Tumor size | Tumor size | Tumor size |
| Tumor density | Tumor density | Histological subtype |
| Surgical access | Surgical access | Surgical access |
| cN PET/CT | cN PET/CT | cN PET/CT |
| Postoperative N | Postoperative N | Postoperative N |
| pTNM | pTNM | pTNM |
| Histological subtype | Histological subtype | FEV1 |
| FEV1 | FEV1 | DLCO |
| DLCO | DLCO | Arrhythmia |
| Stroke | Diabetes | Diabetes |
| Diabetes |
| Lobectomy | Trisegmentectomy | Lobectomy | Trisegmentectomy | Lobectomy | Trisegmentectomy | |
|---|---|---|---|---|---|---|
| Incomplete | 119 | 18 | 119 | 18 | 110 | 17 |
| Complete | 421 | 65 | 421 | 65 | 407 | 63 |
| After propensity score matching | 130 | 65 | 130 | 65 | 126 | 63 |
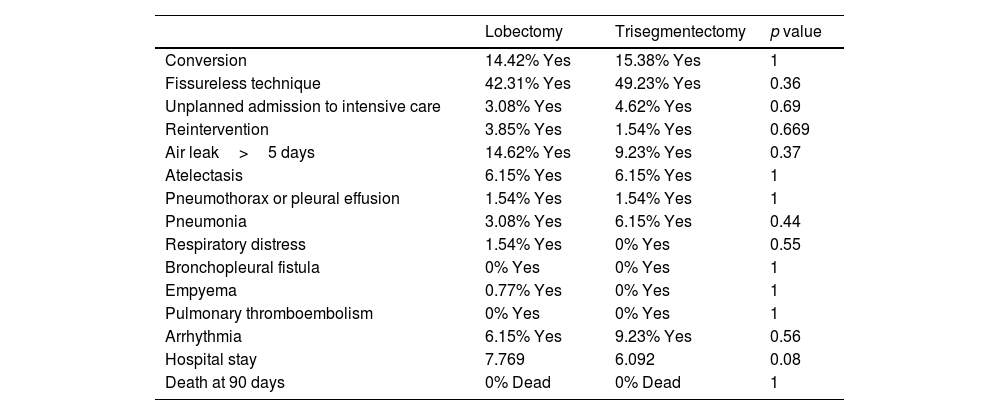
No differences were found between the 2 surgical groups for the 3 analyses in any of the technical aspects (fissureless lobectomy or the need for conversion to thoracotomy), or in postoperative complications or hospital stay. Table 4 provides information on complications after propensity scoring for overall survival. Supplementary Tables 2 and 3 show these results for the other 2 analyses.
Technical Factors, Complications and Hospital Stay of Overall Survival Analysis After Propensity Score Matching.
| Lobectomy | Trisegmentectomy | p value | |
|---|---|---|---|
| Conversion | 14.42% Yes | 15.38% Yes | 1 |
| Fissureless technique | 42.31% Yes | 49.23% Yes | 0.36 |
| Unplanned admission to intensive care | 3.08% Yes | 4.62% Yes | 0.69 |
| Reintervention | 3.85% Yes | 1.54% Yes | 0.669 |
| Air leak>5 days | 14.62% Yes | 9.23% Yes | 0.37 |
| Atelectasis | 6.15% Yes | 6.15% Yes | 1 |
| Pneumothorax or pleural effusion | 1.54% Yes | 1.54% Yes | 1 |
| Pneumonia | 3.08% Yes | 6.15% Yes | 0.44 |
| Respiratory distress | 1.54% Yes | 0% Yes | 0.55 |
| Bronchopleural fistula | 0% Yes | 0% Yes | 1 |
| Empyema | 0.77% Yes | 0% Yes | 1 |
| Pulmonary thromboembolism | 0% Yes | 0% Yes | 1 |
| Arrhythmia | 6.15% Yes | 9.23% Yes | 0.56 |
| Hospital stay | 7.769 | 6.092 | 0.08 |
| Death at 90 days | 0% Dead | 0% Dead | 1 |
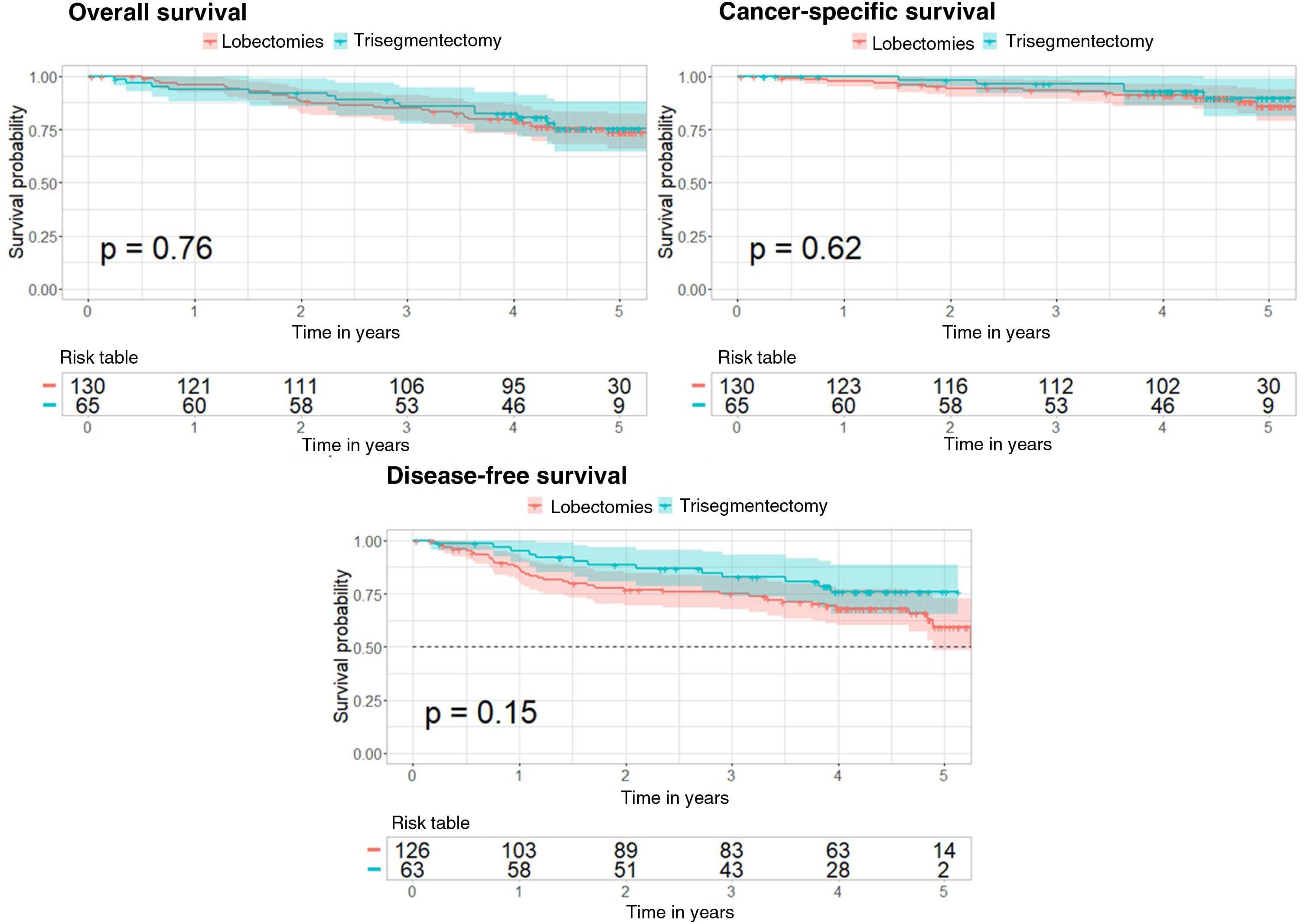
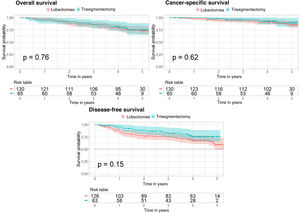
A Cox regression was performed to determine if trisegmentectomy increases the risk of survival, cancer-specific survival, and disease-free survival compared to lobectomy. This Cox regression was conducted with comparable groups obtained from propensity score matching for the 3 analyses. The Kaplan–Meier curve was also applied for the 3 analyses and compared with the log-rank test (Fig. 2). Supplementary Tables 5–7 show the 95% confidence interval for the risk in each case.
In the case of overall survival, the p value obtained is not significant (p=0.76) showing that the type of surgery does not affect the risk of survival. Furthermore, the Kaplan–Meier curves corresponding to the types of surgery (Fig. 2) show no significant differences (log-rank test, p=0.76).
With regard to cancer-specific survival, the results are very similar, with a p value of 0.62 for the Cox regression and a p value of 0.62 in the log-rank test comparing the 2 Kaplan–Meier curves (Fig. 2).
In the case of disease-free survival, the p value of the Cox regression is 0.15 and the p value for comparison of the 2 Kaplan–Meier curves (Fig. 2) is 0.15. These results show that the type of surgery does not affect the risk of recurrence.
Comparable groups determined by propensity score matching were used in the 3 analyses: 291 patients were excluded out of 486 cases with complete data in the first 2 analyses; and 281 were excluded out of 470 cases with complete data in the third analysis. To avoid patient loss, a Cox regression was also performed with all patients using the IPTW method. The Cox regression p value for overall survival was 0.23; for cancer-specific survival, it was 0.73; and for disease-free survival, it was 0.28. These results are consistent with previous reports suggesting that trisegmentectomy does not affect the risk of overall survival or disease-free survival compared to lobectomy.
Disease-free Survival Results for the Complete SetThe analysis of disease-free survival using the set of patients with complete information (597 patients, 517 lobectomies and 80 trisegmentectomies) showed a recurrence rate of 37.52% for lobectomies and 28.75% for trisegmentectomies. This difference was not significant according to Fisher's exact test (p value=0.1359).
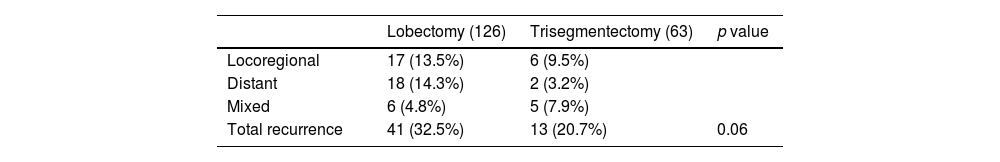
In comparable groups (Table 5) comprising 189 patients (126 lobectomies and 63 trisegmentectomies), recurrence was 32.53% after lobectomy, compared to 20.63% after trisegmentectomy. This difference was not significant according to Fisher's exact test (p value=0.12). It should be noted that, in the lobectomy group, 14.3% of patients had distant recurrence compared to 3.2% in the trisegmentectomy group (p=0.02). If we combine these recurrences with mixed recurrence rates (locoregional+distant), a total of 19% of the patients in the lobectomy group had recurrence compared to 11% in the trisegmentectomy group (p=0.21). Table 5 shows the recurrence rates in both groups after the creation of comparable groups.
Type of Recurrence in 189 Patients (126 Lobectomies and 63 Trisegmentectomies) Included in the Disease-free survival Analysis After Propensity Score Matching.
| Lobectomy (126) | Trisegmentectomy (63) | p value | |
|---|---|---|---|
| Locoregional | 17 (13.5%) | 6 (9.5%) | |
| Distant | 18 (14.3%) | 2 (3.2%) | |
| Mixed | 6 (4.8%) | 5 (7.9%) | |
| Total recurrence | 41 (32.5%) | 13 (20.7%) | 0.06 |
Number of cases of each type of recurrence and percentage of the total number of cases in each group are shown. The p value is that of the Fisher's exact test result.
In this second analysis, patients with tumors measuring between 20mm and 30mm were studied, resulting in a total of 97 patients, of whom 82 underwent lobectomies and 15 trisegmentectomies.
The variables used for the propensity score for the 3 types of analyses are shown in Supplementary Table 4. After propensity score matching, a subset of 45 patients (30 left upper lobectomies and 15 trisegmentectomies) was obtained for the 3 analyses. For the disease-free survival analysis, 3 patients with incomplete data were excluded from the calculation of the propensity score.
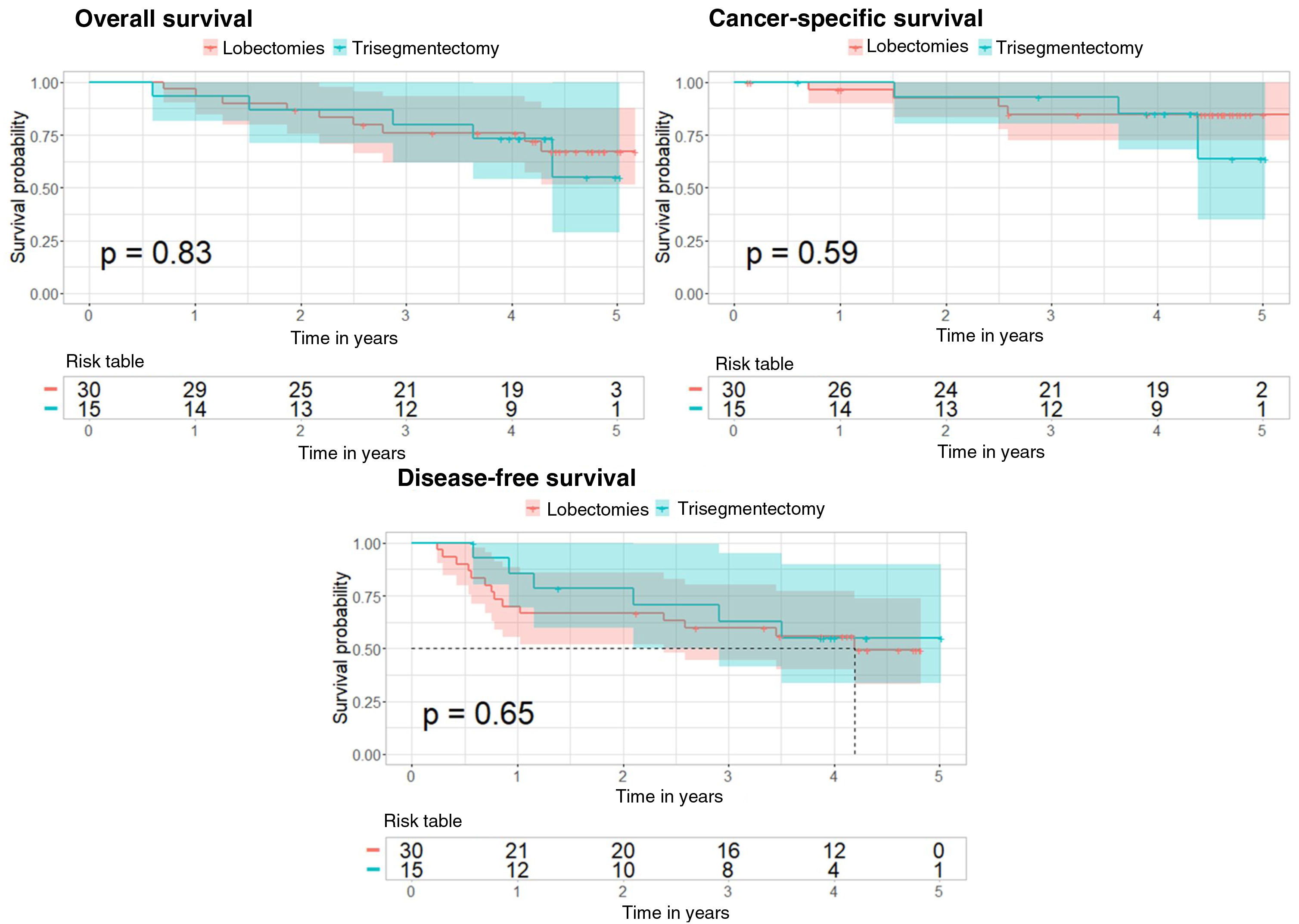
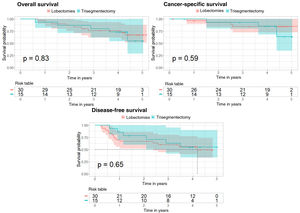
For the overall survival analysis, there is no difference in risk between the 2 types of surgery (p value=0.834 for the Cox regression and p value=0.83 for the log-rank test). For the cancer-specific survival analysis, the Cox regression p value was 0.59, and also 0.59 for the log-rank test. Finally, for the disease-free survival analysis, the p value of the Cox regression was 0.646, and for the log-rank test, it was 0.65 (Fig. 3).
Kaplan–Meier curves after propensity score matching for overall survival, cancer-specific survival, and disease-free survival analyses in the subgroup of patients with tumors measuring 20–30mm. The 95% confidence interval and the p value corresponding to the log-rank test are shown for the 3 analyses.
The results obtained using the IPTW method are also consistent, with p values for the 3 analyses of 0.582, 0.743 and 0.611, respectively.
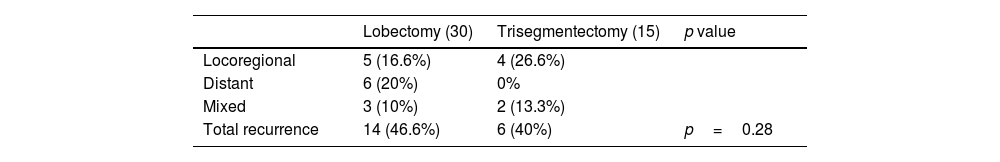
Table 6 summarizes the type of recurrence for each surgical approach. Differences are observed, but they are not statistically significant.
Pattern of Recurrence in 45 Patients (30 Lobectomies and 15 Trisegmentectomies) Included in the Disease-free Survival Analysis After Propensity Score Matching in Subgroup of Patients With Tumors Measuring 20–30mm.
| Lobectomy (30) | Trisegmentectomy (15) | p value | |
|---|---|---|---|
| Locoregional | 5 (16.6%) | 4 (26.6%) | |
| Distant | 6 (20%) | 0% | |
| Mixed | 3 (10%) | 2 (13.3%) | |
| Total recurrence | 14 (46.6%) | 6 (40%) | p=0.28 |
Number of cases of each type of recurrence and percentage of the total number of cases in each group are shown. The p value is that of the Fisher's exact test result.
Lobectomy has been the mainstay of early-stage lung cancer treatment since Ginsberg et al. published their findings in 1995.12 Two more recent clinical trials concluded that survival is similar in N0 patients with peripheral tumors measuring less than 2cm undergoing sublobar resections.
Trisegmentectomy is an intervention that lies midway between segmentectomy and lobectomy. Some authors believe that left upper lobe trisegmentectomy is similar to right upper lobectomy, as the lingula and the middle lobe maintain a similar anatomic relationship with their respective upper segments.3
Some clear differences can be observed in the published literature on segmentectomies compared with trisegmentectomies. First, the quality of the studies on the indications for segmentectomy is high as it is based on 2 randomized clinical trials. In these reports, trisegmentectomies were included in the segmentectomy group, so the indication for tumors measuring less than 2cm and N0 seems clear. In contrast, for studies conducted specifically to compare trisegmentectomy with left upper lobectomy, the literature is based on observational studies and, as such, is not of such high quality.4–8 In a recent meta-analysis9 combining the 5 most important comparative studies on the subject,4–8 trisegmentectomy appeared to be equivalent to left upper lobectomy in terms of survival in disease up to and including stage IB tumors measuring up to 4cm5,7 or even 5cm.6
In our study, we compared overall, cancer-specific and disease-free survival between the 2 types of surgery. It should be noted that differences in survival between the groups may be due to cofactors such as tumor size, tumor density, pTNM, FEV1, DLCO, etc. If one of these cofactors differs significantly between the 2 surgical groups (e.g., tumor density, Table 1), the difference or lack of difference in survival between the 2 types of surgery may be due to the difference in that factor (e.g. tumor density) rather than to the different interventions. To avoid this effect, we applied the 2:1 nearest neighbor technique to create comparable groups. In this way, we ensured that the difference or the absence of difference in survival between the 2 types of surgery was due to the type of surgery and not to the cofactor. The results showed no differences in overall survival, cancer-specific survival and disease-free survival in comparable groups after propensity score matching. We should remember that patient data are lost when the 2:1 nearest neighbor technique is applied, so the results obtained are true for the subset of patients selected after the propensity score. To address this limitation, we completed the statistical analysis using the IPTW technique. This method reduces the possible bias that results from having non-comparable groups.13 Specifically, with the IPTW technique, the comparison of survival is made using all patients, weighing the importance of each patient according to the score obtained in the propensity score. With this analysis, we generated results consistent with those obtained with the 2:1 nearest neighbor technique.
A particularly interesting finding in our cohort was the pattern of recurrence: the lobectomy group showed a greater tendency to distant metastases than the trisegmentectomy group, with 18 patients (14.3%) vs 2 patients (3.2%), respectively (p=0.02). If we add mixed dissemination to the distant dissemination observed in lobectomies versus trisegmentectomies (19% vs 11%; p=0.21), the trend remains higher in the lobectomy group, although statistical significance is lost. Although this relationship and its causes are not known, after analyzing the variables used to obtain the propensity score, no errors were observed that might explain these findings. This difference is also seen in other studies. For example, Aprile et al.7 reported that 20.2% of patients treated with lobectomy had distant metastases compared to 9.4% of patients treated with trisegmentectomy (p=0.067). Zhou et al.8 also identified this difference, but to a lesser degree due to a lower rate of recurrences, probably associated with the histological characteristics of their Asian cohort. In fact, 96.7% of the lobectomy group and 96% of the trisegmentectomy group in Zhou's cohort were adenocarcinomas, compared to 50.4% and 62.6%, respectively, in our patients. Likewise, only 17% of Zhou's cohort had solid tumors compared to >80% of ours. This trend was also clearly observed in the above-mentioned meta-analysis,9 where statistical significance was not reached due to low case numbers. Significance may be reached if our data were added to the patient numbers. In our opinion, this finding is of the utmost importance, and we believe that it should be further evaluated in subsequent research on this type of surgery.
All studies on trisegmentectomies share the limitations of a retrospective design and a low number of patients in stage IB. Although the general conclusion is that this surgery is justified for tumors beyond stage IA, none of the authors included more than 20 cases in stage IB or higher. Our study is similarly limited, since we recruited only 45 patients with tumors measuring more than 2cm (30 lobectomies and 15 trisegmentectomies). Nevertheless, the pattern of recurrence in these patients is surprisingly similar to that of the whole group.
ConclusionsTrisegmentectomy and left upper lobectomy show comparable 5-year survival rates. In our database, recurrence after trisegmentectomy was lower than after left upper lobectomy, while the pattern of recurrence was different for both surgical approaches, with a greater tendency to distant metastasis after left upper lobectomy.
The number of cases with tumors measuring 20–30mm was low but the patterns of recurrence were similar.
For this reason, we believe that trisegmentectomy should be considered as the first surgical option in all these cases.
Funding StatementAll costs related to the start-up and maintenance of the GEVATS database were covered by Ethicon, Johnson & Johnson. The authors had freedom of research and complete control over the study design, the methods used, the outcome parameters and the results, the analysis of data and the production of the written report.
Conflicts of InterestThe authors state that they have no conflict of interests.
We thank Johnson & Johnson for their collaboration in the development of the Spanish VATS Group. We also thank all the clinical documentation managers at each hospital for actively participating in the audit of our study.
Sergio Bolufer (Servicio de Cirugía Torácica, Hospital General Universitario de Alicante, Alicante), Miguel Congregado (Servicio de Cirugía Torácica, Hospital Universitario Virgen Macarena, Sevilla), Marcelo F. Jiménez (Servicio de Cirugía Torácica, Hospital Universitario de Salamanca, Universidad de Salamanca, IBSAL, Salamanca), Sergio Amor-Alonso (Servicio de Cirugía Torácica, Hospital Universitario Quirón salud Madrid, Madrid), Miguel Jesús Arrarás (Servicio de Cirugía Torácica, Fundación Instituto Valenciano de Oncología, Valencia), Ana Isabel Blanco Orozco (Servicio de Cirugía Torácica, Hospital Universitario Virgen del Rocío, Sevilla), Marc Boada (Servicio de Cirugía Torácica, Hospital Clinic de Barcelona, Instituto Respiratorio, Universidad de Barcelona, Barcelona), Isabel Cal (Servicio de Cirugía Torácica, Hospital Universitario La Princesa, Madrid), Ángel Cilleruelo Ramos (Servicio de Cirugía Torácica, Hospital Clínico Universitario, Valladolid), Elena Fernández-Martín (Servicio de Cirugía Torácica, Hospital Clínico San Carlos, Madrid), Santiago García-Barajas (Servicio de Cirugía Torácica, Hospital Universitario de Badajoz, Badajoz), María Dolores García-Jiménez (Servicio de Cirugía Torácica, Hospital Universitario de Albacete, Albacete), José María García-Prim (Servicio de Cirugía Torácica, Hospital Universitario Santiago de Compostela, Santiago de Compostela), José Alberto García-Salcedos (Servicio de Cirugía Torácica, Hospital Universitario 12 de Octubre, Madrid), Juan José Gelbenzu-Zazpe (Servicio de Cirugía Torácica, Complejo Hospitalario de Navarra, Pamplona), Carlos Fernando Giraldo-Ospina (Servicio de Cirugía Torácica, Hospital Regional Universitario, Málaga), María Teresa Gómez Hernández (Servicio de Cirugía Torácica, Hospital Universitario de Salamanca, Universidad de Salamanca, IBSAL, Salamanca), Jorge Hernández (Servicio de Cirugía Torácica, Hospital Universitario Sagrat Cor, Barcelona), Jennifer D. Illana Wolf (Servicio de Cirugía Torácica, Hospital Puerta del Mar, Cádiz), Fernando Ascanio (Servicio de Cirugía Torácica, Hospital Universitario Vall d’Hebron, Barcelona), Unai Jiménez (Servicio de Cirugía Torácica, Hospital Universitario Cruces, Bilbao), Néstor J. Martínez-Hernández (Servicio de Cirugía Torácica, Hospital Universitario La Ribera, Alcira, Valencia), Elisabeth Martínez-Téllez (Servicio de Cirugía Torácica, Hospital Santa Creu y Sant Pau, Universidad Autónoma de Barcelona, Barcelona), Roberto Mongil Poce (Servicio de Cirugía Torácica, Hospital Regional Universitario, Málaga), Francisco Javier Moradiellos-Díez (Servicio de Cirugía Torácica, Hospital Universitario Quirón salud Madrid, Madrid), Ramón Moreno-Basalobre (Servicio de Cirugía Torácica, Hospital Universitario La Princesa, Madrid), (Servicio de Cirugía Torácica, Hospital Universitario Virgen Macarena, Sevilla), Florencio Quero-Valenzuela (Servicio de v, Hospital Virgen de las Nieves, Granada), María Elena Ramírez-Gil (Servicio de Cirugía Torácica, Complejo Hospitalario de Navarra, Pamplona), Ricard Ramos-Izquierdo (Servicio de Cirugía Torácica, Hospital Universitario de Bellvitge, Hospitalet de Llobregat, Barcelona), Eduardo Rivo (Servicio de Cirugía Torácica, Hospital Universitario Santiago de Compostela, Santiago de Compostela), Alberto Rodríguez-Fuster (Servicio de Cirugía Torácica, Hospital del Mar. I M I M (Instituto de investigación médica Hospital del Mar, Barcelona), Rafael Rojo-Marcos (Servicio de Cirugía Torácica, Hospital Universitario Cruces, Bilbao), David Sánchez-Lorente (Servicio de Cirugía Torácica, Hospital Clinic de Barcelona, Instituto Respiratorio, Universidad de Barcelona, Barcelona), Laura Sánchez Moreno (Servicio de Cirugía Torácica, Hospital Universitario Marqués de Valdecilla, Santander), Carlos Simón (Servicio de Cirugía Torácica, Hospital Universitario Gregorio Marañón, Madrid), Juan Carlos Trujillo-Reyes (Servicio de Cirugía Torácica, Hospital Santa Creu y Sant Pau, Universidad Autónoma de Barcelona, Barcelona), Cipriano López García (Servicio de Cirugía Torácica, Hospital Universitario de Badajoz, Badajoz), Juan José Fibla Alfara (Servicio de Cirugía Torácica, Hospital Universitario Sagrat Cor, Barcelona), Julio Sesma Romero (Servicio de Cirugía Torácica, Hospital General Universitario de Alicante, Alicante), Mario Montesinos (Servicio de Cirugía Torácica Lleida), Carme Obiols (Servicio de Cirugía Torácica, Hospital Universitario Mutua Terrasa, Terrasa), Sergi Call (Servicio de Cirugía Torácica, Hospital Universitario Mutua Terrasa, Terrasa), David Gomez de Antonio (Servicio de Cirugía Torácica, Hospital Universitario Puerta de Hierro, Madrid), Silvana Crowley (Servicio de Cirugía Torácica, Hospital Universitario Puerta de Hierro, Madrid), Alberto Cabañero (Servicio de Cirugía Torácica, Hospital Universitario Ramón y Cajal, Madrid), Nicolás Moreno (Servicio de Cirugía Torácica, Hospital Universitario Ramón y Cajal, Madrid), Alvaro Fuentes (Servicio de Cirugía Torácica, Hospital Clínico Universitario, Valladolid), Ana Isabel Triviño (Servicio de Cirugía Torácica, Hospital Universitario Virgen Macarena, Sevilla), Marta López (Servicio de Cirugía Torácica, Hospital Universitario Virgen Macarena, Sevilla) and Florentino Hernando Trancho (Servicio de Cirugía Torácica, Hospital Clínico San Carlos, Madrid).