Solitary pulmonary micronodules (SPMN) characteristically have a diameter of 0.1–0.5cm.
ObjectiveThe aim of this prospective study is to evaluate the surgical approach to SPMN in order to establish the most appropriate treatment.
MethodsBetween January 2007 and June 2011, 146 SPMN patients (94 males and 52 females) were prospectively evaluated. Patients were divided into two groups based on history of malignancy (Group A, 59 patients) and generic risk factors for lung cancer (Group B, 87 patients). After gathering patient information, we proposed surgery or thin-section computed tomography (TSCT) follow-up to both Groups.
ResultsPreference for surgery versus TSCT follow-up was 90% versus 10% in Group A and 78% versus 22% in Group B, respectively. In Group A, we discovered 46 metastases from previous cancer (78%), 8 primary lung cancers (14%) and 5 benign lesions (8%). In Group B, we found 5 metastases (6%), 13 non-small-cell lung cancer (15%) and 69 benign lesions (79%). Statistical analysis revealed a high positive predictive value (PPV=0.9) between total surgical patients versus TSCT follow-up patients.
ConclusionsThe indication for surgery in solitary pulmonary micronodules is aimed at establishing early diagnosis and curing malignant disease. Our study indicates that in patients with previous cancer, surgery is essential. In patients with generic risk for lung cancer, surgical indications should be contemplated more carefully, even though the pulmonary malignancy rate of 21% in Group B seems to indicate the advisability of surgery.
Los micronódulos pulmonares solitarios (MNPS) se caracterizan por un diámetro de 0.1–0.5cm.
ObjetivoEl objetivo de este estudio prospectivo es evaluar el abordaje quirúrgico del MNPS con objeto de establecer el tratamiento más apropiado.
MétodosEntre enero del 2007 y junio del 2011, se evaluó prospectivamente a un total de 146 pacientes con MNPS, 94 varones y 52 mujeres. Se dividió a los pacientes en 2 grupos en función de los antecedentes de enfermedad maligna (grupo A, 59 pacientes) y los factores de riesgo genéricos para el cáncer de pulmón (grupo B, 87 pacientes). Tras obtener información sobre los pacientes, propusimos la cirugía o un seguimiento mediante tomografía computarizada de cortes finos (TCCF) a ambos grupos.
ResultadosLa preferencia por la cirugía frente al seguimiento con TCCF fue del 90% frente al 10% en el grupo A y del 78% frente al 22% en el grupo B, respectivamente. En el grupo A detectamos 46 metástasis de un cáncer previo (78%), 8 cánceres primarios de pulmón (14%) y 5 lesiones benignas (8%). En el grupo B encontramos 5 metástasis (6%), 13 cánceres de pulmón no microcíticos (15%) y 69 lesiones benignas (79%). El análisis estadístico puso de relieve un valor predictivo positivo elevado (VPP=0,9) para el total de pacientes quirúrgicos frente a los pacientes con seguimiento mediante TCCF.
ConclusionesLa indicación para la cirugía en el micronódulo pulmonar solitario tiene como finalidad establecer un diagnóstico precoz y la curación de la enfermedad maligna. Nuestro estudio indica que, en los pacientes con un cáncer previo, la cirugía es imprescindible. En los pacientes con un riesgo genérico de cáncer de pulmón, las indicaciones quirúrgicas deben ser más cuidadosas, aun cuando un 21% de enfermedades malignas en el grupo B parece indicar en cualquier caso la conveniencia de la cirugía.
The probability of detecting a solitary pulmonary nodule (SPN) by means of radiological exam has increased considerably in recent years.1,2 This is due to the use of fine cut ct scans (FCCT), which can show lesions that are no larger than 0.1–0.5cm, thus reducing the number of false images caused by respiratory or cardiac activity. As a result of improvements in CT scan technology, the definition of SPN3,4 as a round-shaped structured lesion with a diameter of ≤3cm has probably become obsolete. In our opinion it is preferable to think of two separate entities: the solitary pulmonary micro-nodule (SPMN), with a diameter of between 0.1 and 0.5cm, and the SPN, with a diameter of between 0.6 and 2cm. Lesions with a diameter greater than 2cm should be regarded as mass and presumably indicative of lung cancer unless proven not to be via histological tests. In fact, Wahidi et al.5 showed the presence of malignancy in between 33% and 64% of the nodules with a diameter of between 1.1 and 2cm and in between 64% and 82% in nodules with a diameter of more than 2cm. A range of diagnostic approaches can be applied to the SPN in order to determine its nature. The spiral CT scan, which highlights the morphological features of the SPN, can establish the benign or malignant potential of the lesion. Lee et al.6 carried out a reconstruction of the solitary pulmonary nodules that were smaller than 1cm, by means of CT cuts of 5mm and 1mm, in 529 patients. These authors pointed out that the nodules were different in terms of size, the presence of calcifications and their consistency depending on whether the CT cut used was 5mm or 1mm. Their conclusion was that both the fine cut and thick cut CT are important for an optimal characterisation of the nodules. The positron emission tomography with 18-F fluorodeoxyglucose (18F-FDG PET) guarantees better contributions to the treatment of the SPN. Christensen et al.,7 in a study of 42 pulmonary nodules with a diameter larger than 7mm, showed a positive qualitative result for 18-F fluorodeoxyglucose (18F-FDG PET) in 24 out of 25 cases of malignant tumours, and in 4 out of 17 benign lesions. These authors described a sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 96%, 76%, 86%, and 93% respectively. In our experience,8 which includes 57 patients with lesions of between 5mm and 9mm, the 18F-FDG PET showed a sensitivity, specificity and accuracy of 95%, 72%, and 82% respectively. The fibrobronchoscopy,9 the endobronchial ultrasound-guided transbronchial biopsy (EBUS-TBB/TBNA),10 the CT led percutaneous pulmonary biopsy (CTPLB)11,12 and video-assisted thoracic surgery (VATS)13 for peripheral SPNs made it possible to obtain a preoperative histological diagnosis and to establish procedures for surgery. Treatment for SPMN remains controversial. Nodules smaller than 5mm do not reveal any specific radiological features which would indicate whether they are benign or malignant. Even minimally invasive techniques (EBUS-TBB/TBNA, CTPLB, VATS) for the detection and diagnosis of an SPMN are not recommended, whatever the location of the latter, due to the size of the nodule. Our clinical experience suggests that minithoracotomy with muscular conservation and manual lung palpation is the best option for locating and removing such a lesion, together with an intraoperative histological assessment. The aim of this study is to define the ideal diagnostic and therapeutic approach for SPMN.
Patients and MethodsFollowing the approval of the ethics committee, between January 2007 and June 2011 146 patients with SPMN, detected by means of a thoracic FCCT, were included in our study. The group was composed of 94 men (64%) and 52 women (36%), with an average age of between 51±9 years (range 41–84). The SPMN were not calcified, did not have an opaque appearance in a frosted glass recipient and presented regular margins. In all the patients the autofluorescence bronchoscopy proved negative for premalignant or malignant lesions, and none of the patients presented either sleep-obstructing apnea or comorbidities. The two groups of patients were defined in terms of the data obtained in the anamnesis, and a different treatment methodology was applied14: (a) group A, patients with antecedents: of malignant disease, in which case the treatment recommended was extirpation by means of muscle-sparing minithoracotomy. In the case of any patient who did not give his or her consent for such a procedure, the approach recommended was thoracic FCCT after 2 months, and (b) group B, patients with general risk factors for lung-cancer (tobacco smoking, advanced age and chronic obstructive pulmonary disease), who were given a choice between resection surgery and thoracic FCCT assessment after 3 months. The FCCT test after 2 or 3 months in group A and B respectively was associated with an examination of the factors which influence the probability of lung-cancer in a pulmonary nodule as proposed by Libby et al.15 Following verification by means of FCCT, the decision to perform surgery in the case of both groups was determined by the persistence and size of the nodule. Group A was made up of 59 patients (40%) with a history of cancer in locations other than the lungs, and aged between 41 and 55, who had not been referred by oncologists. The primary tumours were of the following types: 21 colorectal (36%), 15 kidney (25%), 13 breast (22%), 6 prostate (10%), 3 urinary bladder (5%) and 1 ethmoid (2%). The cancers in question had not presented any extraregional location (M0) and had required adjuvant chemotherapy in 13 patients (22%) on account of N1 lymph node involvement (8 colorectal cancers and 5 breast cancers). The period during which the patients had been free of illness between the previous cancer and the detection of lung cancer had been of 2±1 years. In 16 patients (27%), we examined the KRAS mutations by means of a polymerase chain reaction (PCR), with a view to defining the correlation between the genetic alteration and the lung cancer. Group B was made up of 87 patients (60%), aged between 56 and 84, with an average tobacco consumption of 20 packets/year. A total of 42 patients with COPD (48%) were classified as stage II according to the GOLD strategy.16 Twenty patients (23%) indicated tobacco smoking antecedents of 40 packets/year. In 28 patients (32%), the detection of SPMN was accidental in the thoracic FCCT carried out on account of chest pain and/or acute dyspnoea. The systematic voluntary cancer detection programme via low dosage thoracic FCCT was the means whereby the cancer was detected in 59 patients (68%). This programme, which made it possible for us to examine 463 patients, was originally intended for workers and retired individuals aged ≥50, with no previous diagnosis of bronchogenic cancer, who had a history of continued tobacco smoking of at least 10 years, and had had an acute pneumonia or hemoptsis episode during the last 5 years, in relation to the inhalation of fine dust particulates.
In view of the fact that both surgery and expectant management entail risks, informed consent was obtained from all patients.
Statistical AnalysisAnalysis was carried out using the SPSS 10.0 programme. The data were introduced into the database by means of the programme SPSS Data Entry II (SPSS Inc., Chicago, USA), and were expressed in the form of mean±standard deviation (range of 95%). In each group what was evaluated was the percentage of cases of primary lung cancer and of diagnosed metastases in patients on whom surgery had previously been performed. An analysis was carried out of the risk ratios which served to define the different variable associations and to enable us to determine the ratio of the observed frequencies (χ2 test) of the incidence of malignant disease. The PPV of the entire surgical process and of the FCCT monitoring was determined in both groups. All the values of P that were lower than .05 were considered to be statistically significant, and an IC of 95% was established.
ResultsIn group A, 53 patients (90%) consented to surgical intervention, while 6 patients (10%) opted for thoracic FCCT after 2 months. In accordance with the intraoperative histological examination, the following operations were performed in the surgery subgroup: (a) 45 wedge resections (85%), 43 for metastasis and 2 for non-specific inflammations, and (b) 8 lobectomies (15%) for primary lung-cancer (3 adenocarcinomas, 4 squamous cell carcinomas and one bronchoalveolar cell carcinoma) associated with lymph node dissection. Secondary tumours were one or other of the following types of carcinoma: 15 colorectal (35%), 12 kidney (28%), 11 breast (26%), 4 prostate (9%) and one ethmoidal (2%). The post-operative histopathology confirmed the intraoperative diagnosis. Non-microcytic lung cancers (NMLC) were classified as being at an IA stage (T1aN0M0). The PCR revealed the presence of KRAS mutations (codons 12 and 13) in 11 (3 adenocarcinomas and 8 colorectal cancers) out of 16 patients. In the patients in whom FCCT monitoring was used, the lesion disappeared in 3 cases (50%) and maintained a stable diameter in the other 3 (50%). The latter three patients opted for surgery at that point and wedge resections revealed a recurrence of the previous cancer (2 colorectal carcinomas and one of the urinary bladder. In total, the percentage of malignant disease in group A was 92% (54 patients) and of benign conditions 8% (5 patients).
In group B, 68 patients (78%) chose surgery and 19 (22%) the thoracic FCCT after 3 months. In the surgery subgroup parenchyma extirpations were performed in the following cases: (a) 59 wedge resections (87%), 55 for non-specific inflammations, haemorrhage or focal fibrosis, and 4 for secondary tumours (3 renal and one colorectal carcinoma); (b) 9 lobectomies (13%) for an NMLC (4 adenocarcinomas, 3 squamous cell carninomas and 2 bronchoalveolar cell carcinomas). The postoperative cerebral and abdominal FCCT identified the primary cancer in the patients with pulmonary metastasis, while it was negative for secondary extrathoracic lesions in those with NMLC (T1aN0M0: stage IA). In those group B patients in whom FCCT monitoring was carried out, the following was observed: (a) an increase in the size of the nodule in 4 patients (21%); in these cases a lobectomy was performed for a squamous cell carcinoma in stage IA (T1aN0M0) in the FCCT; (b) the persistence of stable SPMN in 9 patients (47%), who opted for surgery. The wedge resections made it possible to establish the diagnosis of 8 non-specified inflammations or granulomas, and a prostate cancer metastasis; and (c) the disappearance of SPMN in 6 patients (32%). In all, group B presented malignant disease in 21% (18) of the patients, and benign tumours in 79% (69) of the patients.
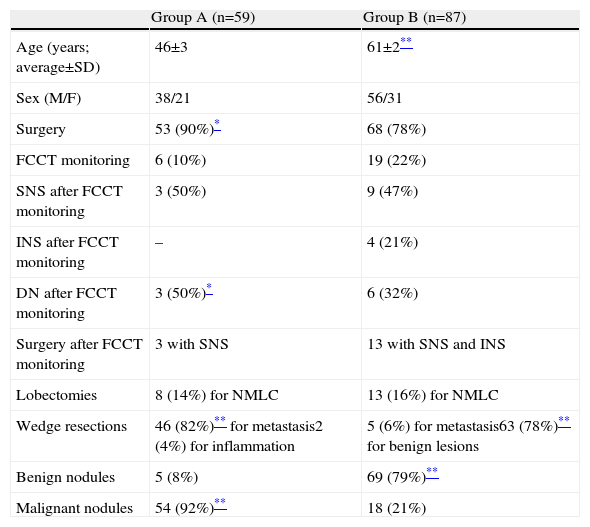
The demographic characteristics of the patients in groups A and B are shown in Table 1.
Clinical Characteristics of the Patients in Group A (Previous Extrapulmonary Cancer) and in Group B (General Risk Factors for Lung Cancer) Included in this Study.
| Group A (n=59) | Group B (n=87) | |
| Age (years; average±SD) | 46±3 | 61±2** |
| Sex (M/F) | 38/21 | 56/31 |
| Surgery | 53 (90%)* | 68 (78%) |
| FCCT monitoring | 6 (10%) | 19 (22%) |
| SNS after FCCT monitoring | 3 (50%) | 9 (47%) |
| INS after FCCT monitoring | – | 4 (21%) |
| DN after FCCT monitoring | 3 (50%)* | 6 (32%) |
| Surgery after FCCT monitoring | 3 with SNS | 13 with SNS and INS |
| Lobectomies | 8 (14%) for NMLC | 13 (16%) for NMLC |
| Wedge resections | 46 (82%)** for metastasis2 (4%) for inflammation | 5 (6%) for metastasis63 (78%)** for benign lesions |
| Benign nodules | 5 (8%) | 69 (79%)** |
| Malignant nodules | 54 (92%)** | 18 (21%) |
INS, increase in nodule size; SD, standard deviation; SNS, stable nodule size; DN, disappearance of nodule; FCCT, fine cut computerised tomography.
Absence of asterisk (*): no statistically significant differences were observed.
All the cancer patients were subjected to radiographic thoracic monitoring during the first 4 months and to whole body FCCT scans after 6 and 12 months during the first year, and then every 12 months from the second year onwards. Whenever a suspicious lesion was observed in the FCCT, the diagnostic study was completed by means of 18F-FDG PET/TC. In patients with pulmonary metastasis targeted chemotherapy was applied. All patients with cancer are still alive and show no signs of the disease 36±2 months after the operation.
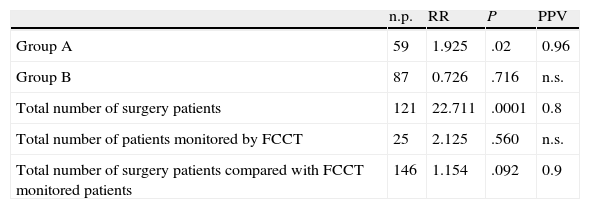
Statistical AnalysisThe analysis of the risk ratio demonstrated the statistically significant association between surgery and follow-up treatment in group A, which confirmed the need for a surgical approach (P=.002; VPP=0.96). This correlation is not statistically significant in group B (P=.716; PPV=ns). The association of the total number of patients on whom surgery was performed in groups A and B was statistically significant (P=.0001; VPP=0.8), which contrasts with what was observed in patients in whom for whom the chosen approach was monitoring (P=.560; PPV=ns). The correlation between the total number of patients on whom surgery was performed and those who were monitored by FCCT did not show statistical significance (P=.092) but the PPV value was high (PPV=0.9), which underlines the fact that surgery was the priority option for treatment (Table 2).
Statistical Analysis of the Different Parameters Evaluated in Group A and Group B Patients.
| n.p. | RR | P | PPV | |
| Group A | 59 | 1.925 | .02 | 0.96 |
| Group B | 87 | 0.726 | .716 | n.s. |
| Total number of surgery patients | 121 | 22.711 | .0001 | 0.8 |
| Total number of patients monitored by FCCT | 25 | 2.125 | .560 | n.s. |
| Total number of surgery patients compared with FCCT monitored patients | 146 | 1.154 | .092 | 0.9 |
n.p., number of patients; n.s., not significant; RR, risk ratio; PPV, positive predictive value.
Our study set out to clarify whether surgery is justified in the treatment of solitary pulmonary micro-nodules, with the aim of ensuring that extirpation does not go beyond the possible benefits that can result from it. Libby et al.17 pointed out that CT monitoring after one year of a nodule with a diameter of <5mm is safe, as the risk of malignancy is low. In 2 lung cancer systematic detection studies the probability of malignancy in a nodule of <5mm has been shown to be less than 1%.1,18 Our results appear to contrast with these findings, as the percentage of malignant and benign lesions was 49% (72 patients) and 51% (74 patients) respectively. What is more, 4 (33%) of the 12 patients with nodules of stable size in the FCCT monitoring presented pulmonary metastasis subsequent to extirpation. This underlines the fact that the time taken for primary or secondary lung cancer to duplicate is variable and the size of the lesion cannot be viewed as a single criterion for malignancy. Due to the cost of the test, the KRAS mutations were studied in only 27% of the patients in the group. Despite the small number of patients, the importance of this test is indisputable as a predictor of pulmonary metastasis. Mascaux et al.19 in a metanalysis of 53 studies on lung cancer, showed that the KRAS mutations were a negative prognostic factor with regard to survival, with a global risk ratio of 1.40 (IC, 1.18–1.65) and of 1.59 (IC, 1.26–2.02) in the adenocarcinomas. The small size of the PSMNs considerably reduces the range of diagnostic and therapeutic options, and that of subsequent clinical decisions. The Fleischner Society20 has proposed guidelines for the treatment of pulmonary nodules of ≤8mm diameter, which have been supported by the American College of Chest Physicians.21 This approach was based on verifications obtained by repeating the CT until up to 24 months after surgery, depending on the presence or absence of risk factors for lung cancer and the stability of the lesion in terms of its size. The increase in the size of the nodule would indicate the need for surgical resection. Our personal opinion, based on the daily clinical practice of lung cancer treatment, is that these strategies can determine the progression of a tumour during the course of observation in such a way that it becomes inoperable. We have also observed that the Fleischer guide, as perceived by radiologists and pulmonologists, leads to differences in the way in which the requisites are fulfilled and to not considering surgery as an option. In group A we discovered 51 malignant lesions (96%) in 53 patients and in group B 13 malignant lesions (19%) in 68 patients who accepted surgery as a first preference. The role of surgery was indisputable in the cases of previous extrathoracic cancer, while it might be debatable where general risk factors are present. In fact, it might be objected that 55 patients (81%) of group B who presented benign lesions were exposed to the risk of possible complications associated with an open surgery resection. It is our opinion that a rapid extirpation of the malignancy should be effected, as this will enhance the quality of life and the chances of survival. Survival of 5 years after the resection of an NMLC in IA or IB is between 63% and 83.7% and 46% and 75% respectively,22,23 thanks to radical surgical intervention. In the case of a solitary metastasis, a period of >36 free of the disease can be considered a favourable prognosis. Metastastectomy guarantees survival of greater than 5 years in the case of melanoma (rate of 16%).24 Although our results showed that a surgical approach had a certain validity and justification in the treatment of SPMN, we believe that the indications to this effect cannot be based on one study at a single centre, but require a general consensus on the part of thoracic surgeons, pulmonologists and oncologists. This consensus implies the recognition that nodules of less than 5mm diameter constitute a clinical entity that must be distinguished from those with a diameter greater than 5mm, each having its distinctive and specific diagnostic and therapeutic problems. Bearing this in mind, the limitation of our study could lie in the fact that it has not taken into account patients with no risk factors for lung cancer.
In conclusion, it remains necessary to carefully examine the treatment of PSMN. Given the small size of the nodules and the impossibility of carrying out a preoperative diagnosis by means of a minimally invasive technique, the patients preferred of were encouraged by clinicians to opt for FCCT monitoring. We believe that surgery cannot be limited to cases where the diameter of the lesion increases in size, as the possibility of malignancy in SPMN remains uncertain, as is also the case with the possibility of the transformation of a localised cancer into one which spreads during the process of monitoring. SPMN which remain unchanged during the first verification with FCCT constitute an indication for extirpation and not a criterion for ruling out surgery. Furthermore, we neither have evidence relating to the social cost of the repetition of the FCCTs, nor any regard to the risks entailed for the patients by exposure to radiation. We hope that our study will serve as a starting point for a randomised multidisciplinary study, designed to establish a set of shared international guidelines for the treatment of SPMN.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Crisci R, Divisi D. Tratamiento clínico del micronódulo pulmonar solitario: un estudio piloto. Arch Bronconeumol. 2013;49:94–8.