Chronic graft-versus-host disease (cGVHD) is a multisystemic disease with high morbidity and mortality that develops as a complication in 30%–70% of allogeneic hematopoietic stem cell (HSCT) transplants1. Bronchiolitis obliterans (BO) is the pulmonary manifestation of cGVHD, and usually presents as fibrosis and scarring of the small distal airway and fixed airflow obstruction1,2. Its clinical presentation includes dyspnea, exercise intolerance, and non-productive cough1,3. Clinical manifestations are non-specific, and many patients are initially asymptomatic, so this disease can be diagnosed late. The incidence in patients receiving allogeneic HSCT is estimated to be 2%–5% and 6% in patients already diagnosed with cGVHD1,4,5, but recent publications suggest that the incidence is on the rise. Thus, the study by Chien et al. showed a prevalence of BO of 5.5% in general, 10% in patients who survived at least one year, and 16% in patients already diagnosed with cGVHD6,7.
The aim of this study was to describe the prevalence, clinical and spirometric characteristics, and survival of patients with GVHD who developed BO in the previous 10 years. The Hematology and Respiratory Medicine Departments of the Hospital de la Princesa have been collaborating for years in the joint follow-up of these patients. We conducted a retrospective observational study of the 289 HSCTs performed at the Hospital de la Princesa between January 2009 and June 2018, and finally selected 42 patients who were diagnosed with BO. The following variables were collected: age at the time of transplantation, sex, baseline hematological disease, lung function at diagnosis of BO and pre- and post-transplant, microbiological isolates, radiological findings, clinical course, involvement of other organs, and mean survival. Differences in survival by sex, microbiological isolates, or involvement of the lung only or of the lung and other organs were evaluated.
Of the 42 patients with BO, 23 were men and 19 were women. The prevalence of BO was 14.8%. The mean age of the patients at transplantation was 48.39 ± 12.74 years. The HSCT was performed mainly for acute myeloblastic leukemia (34.8%), myelodysplastic syndrome (28.3%), and acute lymphoblastic leukemia (13%). In total, 52.4% of the patients were former smokers with a pack/year index of 22.26 ± 13.52. Mean FEV1% was 96.28% ± 11.55 before transplantation; 64.6% ± 24.43 at diagnosis of BO; 66.86% ± 31.08 at 6 months; and 69.37% ± 25.94 at 12 months. Sputum culture was carried out in 19 patients: 10 cases were positive for Aspergillus fumigatus, 7 for Pseudomonas aeruginosa and 2 for Haemophilus influenzae and Stenotrophomonas maltophilia. The findings on chest computed tomography (CT) were: ground glass and bronchiectasis in 56.4%; alveolar infiltrates in 30.8%; air trapping in 15.4%, and peribronchial thickening and peribronchial nodules in 12.8%. No patient showed images consistent with pleuropulmonary fibroelastosis.
In 11 patients, cGVHD was exclusively pulmonary, while involvement of both the lungs and other organs was observed in the remaining patients (74.4%). The most frequently affected organ was the skin, in 24 cases, followed by ocular (20 cases), oral (17 cases), and hepatic (17 cases) manifestations.
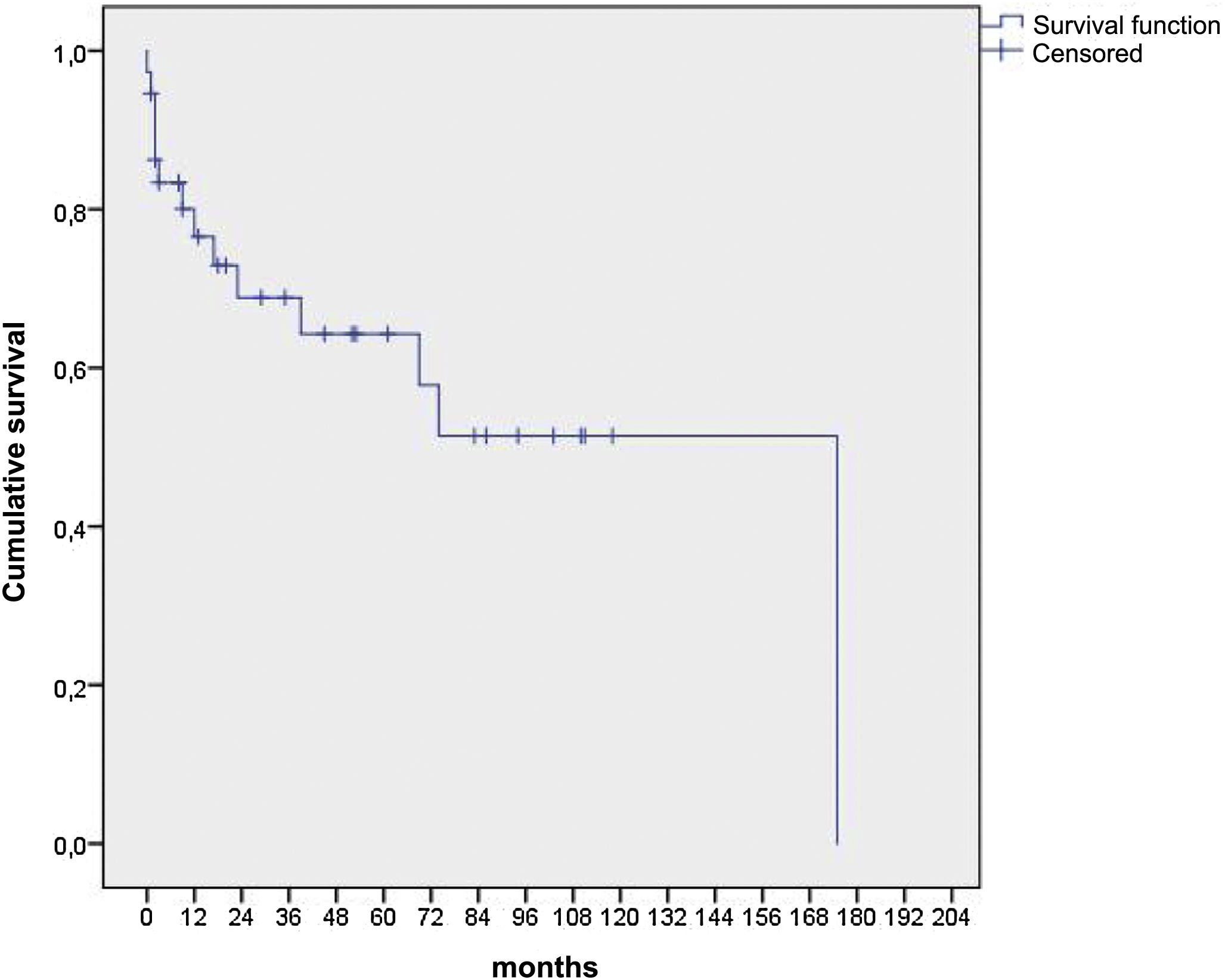
Seventeen patients died, 4 were lost to follow-up, and 24 were still alive at the time of the study. The causes of death were respiratory in 76.4% of the patients, and neurological and digestive in 11.7% each. Fig. 1 shows that the median survival of these patients was 175 months. There were no differences in survival according to sex, microbiological isolates or organ involvement.
In this study, we analyzed the prevalence and clinical characteristics of BO in HSCT. The prevalence of BO in our hospital was 14.8%, which is slightly higher than described in other studies, perhaps due to earlier diagnosis. In previous studies, FEV1% at diagnosis ranged from 40% to 59%, whereas in ours it was 64.6%, suggesting that we may be diagnosing and treating patients with milder involvement. However, mortality was high, and median survival was 175 months. Limitations of our study include particularly the number of patients and the patient inclusion timeline, so it was difficult to analyze all factors that could influence mortality.
In 2015, the National Institutes of Health (NIH) modified their earlier 2009 criteria, specifying that in the presence of any sign of cGVHD, a clinical diagnosis of BO syndrome would be given if all of the following criteria were met: FEV1/FVC < 70% and FEV1 < 75% predicted with decline in FEV1 ≥ 10% in less than 2 years, absence of evidence of active respiratory tract infection at diagnosis from symptoms, chest X-ray or chest CT and microbiological studies (culture of sputum, bronchial aspiration, or bronchoalveolar lavage), and one of the following: presence of air trapping on expiratory acquisition slices, small airway thickening, or bronchiectasis on high-resolution CT of the chest or evidence of air trapping in lung function tests, residual volume (RV) > 120% predicted, or RV/total lung capacity (TLC) > 90%. If the patient already had a diagnosis of GVHD involving any organ, then only the first of the 3 criteria was necessary for the diagnosis of BO. If BO was the only clinical manifestation, a lung biopsy would be required to establish the diagnosis.
The detection of BO requires lung function tests and chest CT tests with expiratory acquisition. Some studies propose performing lung function tests every 3 months in the first 2 years after HSCT. If BO is diagnosed during that period, 3-monthly follow-ups are recommended. In contrast, in patients without BO after 2 years, spirometric monitoring is only proposed in the case of respiratory symptoms1,8.
Recently, the European Society for Blood and Marrow Transplantation published a consensus document containing recommendations for prophylaxis and treatment of GVHD9. FAM therapy is recommended for the management of BO, as follows: inhaled fluticasone 440 μg/2 times daily, azithromycin 250 mg/3 times weekly, and montelukast 10 mg/once daily. The guidelines also recommend discontinuing azithromycin once BO control has been achieved, since the possibility of recurrence of underlying hematological disease associated with prolonged treatments has been described9.
In this study, we conclude that BO is a complication of cGVHD that affects around 15% of patients with allogeneic HSCT and has a mortality rate of up to 45%. It may appear alone or with the involvement of other organs, so management must be multidisciplinary. The role of pulmonologists in the follow-up of these patients is of the utmost importance, and regular functional tests are necessary after the HSCT, along with radiological and microbiological studies in the case of respiratory symptoms, in order to facilitate the early diagnosis and treatment of BO.
Please cite this article as: Martínez-Vergara A, Girón RM, Churruca-Arróspide M, López-Pereira P, Sola-Aparicio E, Aguado-Bueno B. Evolución de los pacientes con bronquiolitis obliterante secundario a trasplante de progenitores hematopoyéticos. Arch Bronconeumol. 2021;57:664–666.