Scant evidence is available on whether dependency for basic (BADL) or instrumental (IADL) activities of daily living can be predictors of mortality after severe COPD exacerbation (COPDE). In addition, it is as yet unclear whether the inclusion of this parameter in a multidimensional score can improve the prediction of mortality.
MethodologyProspective cohort study, with follow-up of patients discharged after COPDE and multivariate analysis of clinical-demographic and dependency variables (Barthel and Lawton and Brody indices) as predictors of mortality. Three scores were generated (including or not including dependency for BADL and IADL) that were compared with each other and with other commonly used multidimensional indices (BODEx, ADO, DOSE, CODEx).
ResultsIn total, 247 patients were included, 112 (45%, 3); and 195 (72.4%) had some dependency for BADL and IADL. Survival was 631.7 (258.8) days, 95% confidence interval (95% CI), 60–912 days. Fifty-four (21.9%, 95% CI 17–27) patients died. Age > 60 years, FEV1 < 50% and Charlson score ≥ 3 were independent predictors in the 3 models generated. Dependency for BADL and IADL were predictors in each of the models in which they were included. The score that included the dependency for BADL presented the best predictive capacity (area under the curve 0.818, 95% CI 0.757–0.879). Stratification into tertiles differentiated groups with a higher risk of death from the beginning of the follow-up (P < .01).
ConclusionsDependence for activities of daily living, especially the most elementary ones, is an independent predictor of mortality after a severe COPDE that is comparable to clinical variables. Its inclusion in multidimensional scores clearly improves predictive capacity.
Apenas existe evidencia si la dependencia para las actividades básicas de la vida diaria (ABVD) o instrumentales (AIVD) son predictores de mortalidad tras una agudización grave de EPOC (AEPOC). Además, no se ha evaluado si su inclusión en un score multidimensional puede mejorar esta predicción.
MetodologíaEstudio prospectivo de cohortes, con seguimiento de pacientes tras un alta por AEPOC y análisis multivariante de variables clínico-demográficas y de dependencia (índices de Barthel y de Lawton y Brody) predictoras de mortalidad. Se generaron 3 scores (con/sin dependencia para ABVD y AIVD) que se compararon entre ellos y con otros índices multidimensionales habituales (BODEx, ADO, DOSE, CODEx).
ResultadosSe incluyó a 247 pacientes, 112 (45%,3) presentaron alguna dependencia para ABVD y 195% (72,4%) para AIVD. Supervivencia 631,7 258,8 días, intervalo de confianza del 95% (IC del 95%), 60-912. Fallecieron 54 (21,9%, IC del 95%, 17-27) pacientes. La edad > 60 años, el FEV1 < 50% y puntuación en Charlson ≥ 3 fueron predictores independientes en los 3 modelos. La dependencia para las ABVD y las AIVD lo fueron en los modelos en los que se incluyeron estas variables. El score que incluía la dependencia para las ABVD presentó la mejor capacidad predictiva área bajo la curva 0,818; IC del 95%, 0,757-0,879) y su estratificación en terciles permitió diferenciar grupos de mayor riesgo desde el inicio del seguimiento (p < 0,01).
ConclusionesLa dependencia para las actividades de la vida diaria, especialmente las más elementales, es un predictor independiente de mortalidad tras una AEPOC grave, comparable con variables clínicas. Su inclusión en scores multidimensionales mejora de forma clara su capacidad predictiva.
Chronic obstructive pulmonary disease (COPD) is one of the most common respiratory diseases. It is associated with high morbidity and mortality, and a significant social and economic burden that is expected to worsen in coming decades1–3, and characterized by chronicity and exacerbations that contribute to a significant deterioration of health, affect the progression of the disease, and increase the risk of death2–4. In patients with advanced disease, the course of COPD is often complex, heterogeneous, and difficult to predict in some cases, making it difficult to establish an accurate prognosis and to determine the point at which palliative care should progressively begin to replace conventional treatment4,5.
Many different studies have focused on identifying reliable predictors of mortality, although the available evidence varies widely, having been obtained from studies with different methodologies, follow-up times, and criteria for patient inclusion (stable phase or after hospitalization)4,5. After an exacerbation, prognostic variables described so far can be grouped into patient characteristics before admission (age, comorbidities, dyspnea, functional capacity, malnutrition, and previous hospitalizations), or clinical severity at admission or during hospitalization5,6–13. However, as the predictive capacity of these variables is limited, the prognostic validity of the combination of several multidimensional indices formed by combinations of these factors has been investigated5,13. The most widely accepted are BODE, BODEx, ADO, DOSE, and CODEx, which include variables such as age, the degree of bronchial obstruction and dyspnea, nutritional status, exercise tolerance, the frequency of previous severe exacerbations, the number of significant comorbidities, or persistent smoking habit4,13–18. Although their value is limited5, the use of these indices is recommended in most COPD guidelines2,3. Recently, a consensus document from the European and American respiratory medicine societies suggested that comparative studies between the various prognostic indices were now priority area for research19.
Activities of daily living (ADL) are among the main elements considered when measuring the health and quality of life of the population20–25. The capacity of patients to live independently within the community must also be determined in order to adapt their care situation and establish priorities for intervention. In contrast to other diseases, surprisingly little attention has been paid to the predictive capacity of dependency for performing ADL in COPD, and the results of the few studies analyzing this factor have been heterogeneous6,7,12. There is as yet no evidence to show the predictive power of functional capacity determined by ADL, which include basic tasks (BADL) of self-care (eating, bathing, dressing, mobility, etc.) and instrumental tasks (IADL) in the immediate environment (shopping, preparing food, cleaning the house, etc.). Moreover, these variables have not been included in any of the prognostic indices developed to date5.
The aim of this study was to evaluate the predictive capacity of dependency for BADL and IADL for mortality after hospitalization for COPD exacerbation, and to analyze if the inclusion of these indices in a multidimensional score would improve this predictive power, in comparison with accepted indices, such as BODEx, ADO, DOSE, and CODEx.
MethodologyStudy design and scopeThis was a prospective cohort study of consecutive patients with a first admission for a primary diagnosis of COPD exacerbation during the study period in the pulmonology department of a third-level hospital throughout 2017. We did not include patients who refused to participate or in whom COPD was ruled out due to failure to meet all the diagnostic criteria, or who received an alternative diagnosis to COPD exacerbation, such as pulmonary embolism, pneumonia or other significantly decompensated cardiorespiratory conditions2,3. Materials and methods have been published elsewhere26,27. The study was approved by the Research Ethics Committee of Galicia (file 2016/524).
Data collectionClinical and demographic variablesTo summarize, between day 3 and day 4 of admission, healthcare personnel and a trained social worker systematically collected demographic, clinical and social data by reviewing the electronic medical record and interviewing the patient and their caregivers. The following data were collected: demographic data, height, weight, active consumption of tobacco, immunizations, number of severe exacerbations, blood test results at admission, number and type of comorbidities according to the Charlson index28, Goldberg anxiety (score ≥ 4) and depression (score > 2) scales29, disease impact using the COPD Assessment Test (CAT) questionnaire30, level of dyspnea at baseline (modified Medical Research Council [mMRC] scale), lung function (FEV1), need for continuous home oxygen therapy or non-invasive ventilation, and medical treatment recommended at discharge.
Dependency variablesAbility to perform BADL at baseline (prior to the exacerbation) was determined using the Barthel index31 and IADL was assessed with the Lawton and Brody index32. Barthel assesses 10 activities (feeding, bathing, dressing, grooming, toilet use, bowels, bladder, mobility on level surfaces, stairs, and transfers). The values assigned to each activity are based on the time and the amount of physical help required, or if the patient performs the activity (0, 5, 10, or 15 points). The range of possible values is between 0 and 100. Between 20 and 60 points indicates severe dependency, between 61 and 90 moderate, between 91 and 99 mild, and 100 independence31. The Lawton and Brody index evaluates 8 items (ability to use the phone, shopping, food preparation, housekeeping, laundry, mode of transportation, responsibility for own medication, and ability to handle finances), to which a number is assigned, 1 (independent) or 0 (dependent), the final score being the sum of all the answers. The total score is measured from 0 to 8, from 0, absolute dependency, to 8, total independence32.
Follow-upAt the time of the analysis, all patients had been followed up for a minimum of 500 days or until death by monthly monitoring of their electronic medical records. Possible cause of death was also recorded from these reviews. In case of doubt or lack of information, the patient or family was contacted.
Statistical analysisSurvival time was defined as the interval between the date of hospital discharge and death or completion of follow-up among survivors. No patients were lost to follow-up. Quantitative variables were expressed as mean and standard deviation (SD), and qualitative variables as number and percentage, including the 95% confidence interval (95% CI) for the incidence of deaths. The median follow-up time was also calculated.
Kaplan-Meier curves and logarithmic rank tests were used to compare all qualitative time-dependent variables in the bivariate analysis, and the Cox proportional hazards analysis was used to compare quantitative variables. All numerical variables were dichotomized (Table 1) according to the cutoff point at which a significant increase in mortality was observed in the standard classification systems (mMRC, CAT, FEV1 according to GOLD, and dependency according to Barthel and Lawton and Brody) or in the contingency table created for the other variables (age or number of hospitalizations for exacerbations). Using all the variables that showed an association with p ≤ 0.10, plus the gender variable, a multivariate analysis was performed using different Cox regression models with fixed covariates. Several models were created: the first included dependency for BADLs (model 1), the second included dependency for IADL (model 2), and the third without any dependency variables (model 3). Results were expressed by their p value and the hazard ratio (HR) with its 95% CI, and statistical significance was set at p < 0.05.
Description of the variables included in the total sample and non-survivors/survivors during the follow-up period and comparison between the variables.
| Total (N = 247) | Deaths (N = 54) | Survivors (N = 193) | p | |
|---|---|---|---|---|
| Male sex (%) | 189 (76.5) | 43 (79.6) | 146 (75.6) | 0.77 |
| Age (years)a | 68.8 ± 9.6 | 72.1 ± 7.9 | 67.6 ± 9.9 | 0.04 |
| Age > 60 years | 204 (82.6) | 52 (96.3) | 152 (78.8) | 0.01 |
| BMI (kg/m2)a | 27.5 ± 6.4 | 25.7 ± 6.2 | 28.01 ± 6.2 | 0.01 |
| BMI > 21 kg/m2 (%) | 49 (19.8) | 16 (29.6) | 33 (17.1) | 0.03 |
| Active smoker (%) | 96 (38.9) | 15 (27.8) | 81 (42.0) | 0.07 |
| Pack-year indexa | 54.2 ± 29.1 | 55.1 ± 29.5 | 54.3 ± 35.7 | 0.93 |
| No. of admissions for exacerbation in previous yeara | 0.7 ± 1.1 | 1.17 ± 1.4 | 0.6 ± 1.0 | 0.03 |
| ≥ 2 admissions for exacerbation in previous year(%) | 49 (19.8) | 17 (31.5) | 32 (16.6) | 0.06 |
| Flu vaccination previous year (%) | 205 (83.0) | 44 (81.5) | 161 (83.4) | 0.75 |
| Prior pneumococcal vaccination (%) | 135 (54.7) | 27 (50.0) | 108 (56.0) | 0.46 |
| Number of eosinophils in blood (total/μl)a | 110 ± 225 | 94 ± 117 | 117 ± 250 | 0.53 |
| CAT scorea | 18.8 ± 7.2 | 20.5 ± 7.0 | 18.4 ± 7.3 | 0.04 |
| CAT score > 10 (%) | 214 (86.6) | 50 (92.6) | 164 (85.0) | 0.10 |
| Dyspnea according to mMRCa | 2.2 ± 0.8 | 2.7 ± 0.7 | 2.1 ± 0.7 | < 0.01 |
| Dyspnea according to mMRC > 2 (%) | 90 (36.4) | 30 (55.6) | 60 (31.1) | < 0.01 |
| FEV1 value (% predicted)a | 42.1 ± 14.2 | 35.6 ± 11.5 | 43.9 ± 14.0 | < 0.01 |
| FEV1 < 50% predicted (%) | 177 (71.4) | 48 (88.9) | 126 (66.3) | < 0.01 |
| Charlson indexa | 1.7 ± 0.9 | 1.9 ± 0.9 | 1.7 ± 0.8 | 0.10 |
| Charlson index score ≥ 3 (%) | 44 (17.8) | 15 (27.8) | 126 (66.3) | 0.04 |
| Goldberg questionnaire (total)a | 5.6 ± 3.9 | 6.04 (± 3.7) | 5.6 ± 3.9 | 0.46 |
| Anxiety according to Goldberg questionnaire (%) | 110 (44.0) | 24 (45.3) | 86 (44.6) | 0.97 |
| Depression according to Goldberg questionnaire (%) | 129 (52.4) | 34 (64.2) | 95 (49.2) | 0.04 |
| Treatment with inhaled corticosteroids | 154 (63%) | 38 (71%) | 114 (60%) | 0.16 |
| Continuous home oxygen therapy (%) | 99 (40.4) | 33 (62.3) | 66 (34.4) | < 0.01 |
| Home non-invasive ventilation (%) | 42 (16.7) | 11 (20.4) | 31 (16.1) | 0.26 |
| Barthel index scorea | 88.7 ± 17.3 | 79.9 ± 19.6 | 91.2 ± 15.8 | < 0.01 |
| Dependency according to Barthel (any) (%) | 112 (45.3) | 41 (75.9) | 71 (36.8) | < 0.01 |
| Lawton and Brody scorea | 4.8 ± 2.4 | 3.6 ± 1.8 | 5.2 ± 2.4 | < 0.01 |
| Dependency according to Lawton-Brody (%) | 195 (72.9) | 51 (94.4) | 144 (74.6) | < 0.01 |
A prognostic score was constructed for the different models (score 1, score 2, and score 3), assigning a value (depending on the HR) to each of the variables taking into account the magnitude of the association with mortality on the Cox regression analysis.
Receiver operating characteristic (ROC) curves were calculated, comparing the area under the curve (AUC) of the C statistic for the 3 different scores, which were also compared with other prognostic indices that could be calculated (BODEx, CODEx, ADO, and DOSE) from the available variables, and their significance was analyzed using the DeLong method. Kaplan–Meier survival curves were also constructed between the number of events and the score with best predictive capacity. The analysis was carried out using the IBM SPSS Statistics package, version 21 (IBM Corporation, Armonk, New York), and R statistical software, version 4.3 (R Core Team, 2017).
ResultsDescription of the seriesA total of 247 patients were included, 189 (76.5%) men, with a mean age of 68.8 (SD 9.6) years. The values of the variables collected are shown in Table 1. Survival time of the entire series was 631.7 (258.8) days, median 712, and 95% CI 60-912 days. Survivors were followed up for 744.2 (136) days, median 771, and 95% CI 516-915 days.
Overall, 54 patients (21.9%, 95% CI, 17–27) died during follow-up, 38 (15.4%, 95%, CI 11–20) of whom died in the first year of follow-up. Mean treatment duration of patients who died was 129.1 ± 109.6 months. No patients were lost to follow-up.
Reliable information on the cause of death was available for 52 of the 54 patients. In 29 (55%) patients, death was due exclusively to refractory decompensated chronic respiratory failure associated with an exacerbation or advanced stage COPD; 5 (9.6%) also had significant heart failure, 3 (6%) pneumonia, 10 (19.2%) advanced cancer (6 lung), 3 (6%) had gastrointestinal disease (2 bowel obstruction of uncertain cause and 1 intestinal ischemia), and 2 (3.8%) stroke.
Variables that predict mortalityTable 1 shows the results of the bivariate analysis. The variables that differed between non-survivors and survivors were: age, low BMI, number of severe exacerbations in the previous year, FEV1, dyspnea grade, CAT score, depression according to Goldberg score, Charlson score, and dependency for BADL and IADL. Dichotomous variables associated with a p ≤ 0.10 and the gender variable were used to create several multivariate models, which are shown in Table 2. Independent predictors were: age > 60 years, FEV1 < 50%, and a Charlson index score ≥ 3 in all 3 models; BMI ≤ 21 in models 1 and 2; home oxygen in models 2 and 3; and dyspnea grade > 2 in model 3 only. Dependency for BADL and IADL were predictors of mortality in all of the models in which they were included.
Multivariate predictive models of mortality and score assigned to each variable included in the scores.
| Model 1 (score 1) | Model 2 (score 2) | Model 3 (score 3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR: | 95% CI | p | Assigned score | HR: | 95% CI | p | Assigned score | HR: | 95% CI | p | Assigned score | |
| Male sex | 0.85 | 0.40–1.71 | 0.69 | – | 0.81 | 0.38–1.72 | 0.58 | – | 0.85 | 0.39–1.83 | 0.68 | – |
| Age > 60 years | 3.96 | 1.01–17.49 | 0.04 | 4 | 4.15 | 1.01–18.04 | 0.04 | 4 | 4.87 | 1.12–20.10 | 0.03 | 5 |
| BMI ≤ 21 kg/m2 | 2.53 | 1.20–5.04 | 0.01 | 2 | 1.95 | 1.01–5.04 | 0.04 | 2 | 1.81 | 0.91–3.59 | 0.09 | – |
| Active smoking | 0.74 | 0.45–1.76 | 0.74 | – | 0.79 | 0.41–1.54 | 0.50 | – | 0.73 | 0.37–1.40 | 0.34 | – |
| ≥ 2 admissions for exacerbation in previous year (%) | 0.91 | 0.48–1.71 | 0.77 | – | 0.96 | 0.50–1.82 | 0.90 | – | 1.03 | 0.54–1.97 | 0.91 | – |
| CAT score > 10 | 0.86 | 0.40–1.82 | 0.69 | – | 0.88 | 0.29–2.63 | 0.82 | – | 1.11 | 0.37–3.28 | 0.84 | – |
| Dyspnea according to mMRC > 2 (%) | 1.56 | 0.82–2.93 | 0.17 | – | 1.59 | 0.87–2.91 | 0.12 | – | 1.84 | 1.01–3.37 | 0.04 | 2 |
| FEV1 < 50% predicted | 3.48 | 1.33–5.04 | 0.01 | 3 | 3.10 | 1.26–7.65 | 0.01 | 3 | 2.90 | 1.17–7.13 | 0.02 | 3 |
| Charlson score ≥ 3 | 2.05 | 1.02–4.15 | 0.04 | 2 | 2.12 | 1.07–4.19 | 0.03 | 2 | 2.18 | 1.1–4.33 | 0.02 | 2 |
| Depression according to Goldberg questionnaire (%) | 1.22 | 0.67–2.49 | 0.51 | – | 1.37 | 0.74–2.54 | 0.31 | – | 1.28 | 0.70–2.35 | 0.42 | – |
| Continuous home oxygen therapy | 1.30 | 0.70–2.45 | 0.43 | – | 1.82 | 1.02–3.32 | 0.04 | 2 | 1.95 | 1.07–3.56 | 0.02 | 2 |
| Any dependency according to Barthel Index | 3.95 | 1.88–8.31 | < 0.01 | 4 | NI | NI | NI | – | NI | NI | NI | – |
| Any dependency according to Lawton and Brody | NI | NI | NI | – | 3.82 | 1.12-12.94 | 0.03 | 4 | NI | NI | NI | – |
NI: not included.
The results of these 3 models were used to create 3 scores, assigning each a value similar to the HR for each variable that was independently associated with the prediction of mortality.
Fig. 1 is a graphical representation of the AUC of all predictive scores calculated. The accompanying table shows the AUC with its 95% CI and p calculated using the DeLong test, when the scores that include dependency (scores 1 and 2, in columns) are compared with those that do not include any dependency variable (score 3, BODEx, CODEx, ADO, DOSE, in the rows). The AUC of score 1 was significantly better than that of all other scores or indices included, while score 2 was better only for BODEx and DOSE. The p value between the AUC of score 1 and score 2 was 0.04.
Graphical representation of the areas under the ROC curve for each of the scores and prognostic indices and comparison (p-value) of the scores that include variables of dependency (scores 1 and 2, in columns) with those that do not include (score 3, BODEx, CODEx, ADO and DOSE, in the rows).
Score 1: age > 60 years (+4), body mass index ≤ 21 (+2), FEV11 < 50% (+3), Charlson score ≥ 3 (+2), any dependency according to Barthel index (+4). Possible score: 0–15
Score 2: age > 60 years (+4), body mass index ≤ 21 (+2), FEV1 < 50% (+3), Charlson score ≥ 3 (+2), home oxygen therapy (+2), any dependency according to Lawton and Brody index (+4). Possible score: 0–17.
Score 3: age > 60 years (+5), dyspnea according to mMRC > 2 (+2), FEV1 < 50% (+3), Charlson score ≥ 3 (+2), domiciliary oxygen (+2). Possible score: 0–14.
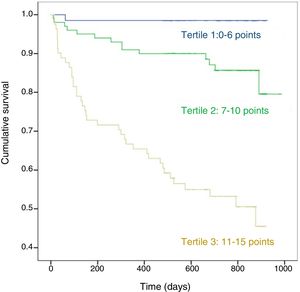
Fig. 2 shows the Kaplan-Meier survival curve for score 1 stratified in tertiles: from the start of follow-up, differences in the 3 groups can be clearly distinguished with marked differences in the probability of death (p < 0.01).
DiscussionThis study provides new information by demonstrating that limitation for performing ADLs, especially the most basic activities, is a powerful predictor of mortality in the months following hospitalization for COPD exacerbation that is equivalent to age and superior to clinical variables, such as obstruction or dyspnea grade, low weight, and number of comorbidities. It is also the first study to demonstrate that the inclusion of this variable in a mortality score following a severe exacerbation can significantly improve the prognostic classification of these patients.
In COPD, existing evidence on the capacity of functional dependency to predict mortality is very limited33. Almagro et al.7 and Sanjaume et al.12 described 2 small cohorts, in which they found that limitation in BADL measured by the Katz scale, which reflects dependency for 7 sociobiological functions also included in the Barthel index, is a predictor of mortality following a severe exacerbation in bivariate analyses only, but not when adjusted for other variables included in the BODEx index or factors such as physical activity and co-morbidities. Connors et al.6, in a study published over 20 years ago, found that BADL dependency measured by this same index was an independent predictor, especially in the case of limitation for at least 2 activities. Similarly, in this study we found that the percentage of deaths in the follow-up period after an exacerbation was 9.6%, 25%, 36%, and 47% (p < 0.01), according to zero, mild, moderate, or severe dependency on the Barthel index, respectively (data not shown). This would mean that the greater the degree of dependency, the higher the likelihood of death. However, in order to simplify scoring, we chose not to stratify for the degree of BADL dependency, despite the fact that this variable has shown great predictive power, both individually and when included in a multidimensional score.
To our knowledge, no previous studies have analyzed the capacity of limitations in IADL for predicting mortality in COPD patients, an association that was revealed in our study. However, although the individual association of IADL with dependency appears similar to that detected for BADL, when it is combined in a multidimensional score its predictive capacity is less than for BADL, although it is superior to some of the previously validated indices, such as BODEx or DOSE. IADL are more dependent on sociocultural factors or gender32, and these factors may have influenced these findings. In addition, when both indices were included in the multivariate predictive models (Barthel and Lawton and Brody), the results are very similar to those described in model 1 (data not shown), which suggests that limitation for BADL is a better predictor, and no added value is gained from the evaluation of both BADL and IADL.
The other predictive variables detected in the different models (age, comorbidities, lung function, dyspnea, low BMI, previous severe exacerbations) were also identified in other studies and are included in many known indexes, including those used in this study4,5,13–18. It is striking that in our study, the score that does not include dependency for ADL (score 3) seems to be a better predictor than those already described, although this may be because it is based on the analysis of the factors that predict mortality in the sample itself.
Our study has some limitations. The first is that it was performed in a single center and with a limited number of cases. This may restrict its external validity, and our findings must be confirmed in multicenter studies with larger sample sizes. However, our study population does not appear to differ much from those found in other centers or countries, as it reflects clinical and demographic characteristics similar to those described in large national and international audits34. Moreover, the number of deaths adjusted for follow-up time and cause was similar to figures reported in other Spanish series7,12,35. In the absence of questionnaires to determine COPD-specific functional capacity validated for the aims of our study33, we used those recommended by the health authorities36,37. This also allowed us to make comparisons with other chronic diseases and to demonstrate the utility of questionnaires that do not focus solely on dyspnea as an essential component of ADL limitation, and may therefore provide a more comprehensive assessment of the general status of the patient, because their results are also related to changes in other areas, such as cognitive, sensory, or musculoskeletal factors, which can be key in the prediction of events in chronic patients5,13,21. Furthermore, collecting information during hospitalization on the stable phase situation of the patient a few weeks previously is prone to inaccuracies.
We believe, however, that our study has some strengths, including its design, the consecutive sampling for 1 year, the assessment of dependency by expert personnel, and the absence of patients lost to follow-up. Our results indicate that evaluating the patient's functional capacity on the basis of a single question instead of measuring all indices, and eliminating the need for intermediate values by dichotimising the remaining variables, make this score very easy to use. Furthermore, the score does not require other functional tests, such as those required by BODE or other recent proposals14,38 that may be unavailable or unevaluable at the time of admission of many patients, and generates evidence on the usefulness of variables of daily life that differ from commonly used clinical parameters. Stratification in tertiles is useful to differentiate very clearly from the start of follow-up 3 groups with different probabilities of death, which can be of help in decision-making at the time of recommending discharge of a patient after a severe exacerbation.
In conclusion, ADL limitation, especially for the most basic activities, is an independent predictor of mortality following a severe COPD exacerbation, and its inclusion in multidimensional scores improves their predictive ability. These results must be validated in multicenter studies and other populations of COPD patients.
Conflict of interestsThe authors state that they have no conflict of interests.
This study was funded by grants obtained from SEPAR (grant 456/2017), FIS-ISCII PI18/01317 (FEDER) and the unconditional collaboration of the company Menarini.
Please cite this article as: Fernández-García S, Represas-Represas C, Ruano-Raviña A, Botana-Rial M, Martínez-Reglero C, Villar AF. La dependencia para actividades como factor predictor de mortalidad tras una hospitalización por una agudización de enfermedad pulmonar obstructiva crónica. Arch Bronconeumol. 2020;56:291–297.