This present paper describes the method and the organization of the study known as the COPD History Assessment In SpaiN (CHAIN), whose main objective is to evaluate the long-term natural history of a chronic obstructive pulmonary disease (COPD) patient cohort from a multidimensional standpoint and to identify clinical phenotypes, in comparison with another non-COPD control cohort.
Patients and methodsCHAIN is a multicenter, observational study of prospective cohorts carried out at 36 Spanish hospitals. Both cohorts will be followed-up during a 5-year study period with complete office visits every 12 months and telephone interviews every 6 months in order to evaluate exacerbations and the vital state of the subjects. The recruitment period for cases was between 15 January 2010 and 31 March 2012. At each annual visit, information will be collected on: (i) clinical aspects (socio-economic situation, anthropometric data, comorbidities, smoking, respiratory symptoms, exacerbations, quality of life, anxiety-depression scale, daily life activities, treatments); (ii) respiratory function (spirometry, blood gases, hyperinflation, diffusion, respiratory pressures); (iii) BODE index (main study variable); (iv) peripheral muscle function, and (v) blood work-up (including IgE and cardiovascular risk factors). In addition, a serum bank will be created for the future determination of biomarkers. The data of the patients are anonymized in a database with a hierarchical access control in order to guarantee secure information access. The CHAIN study will provide information about the progression of COPD and it will establish a network of researchers for future projects related with COPD.
El presente trabajo describe el método y la organización del trabajo del estudio COPD History Assessment in SpaiN (CHAIN), cuyo objetivo principal es evaluar a largo plazo la historia natural de una cohorte de pacientes con enfermedad pulmonar obstructiva crónica (EPOC) desde un punto de vista multidimensional y la identificación de fenotipos clínicos comparándola con otra cohorte control sin EPOC.
Pacientes y métodoCHAIN es un estudio observacional multicéntrico de cohortes prospectivas realizado en 36 hospitales españoles. Ambas cohortes se seguirán durante un periodo de 5 años con visitas completas cada 12 meses y controles telefónicos cada 6 meses para valorar las exacerbaciones y el estado vital del sujeto. El periodo de reclutamiento de casos se realizó entre el 15 de enero de 2010 y el 31 de marzo de 2012. En cada visita anual se recoge información sobre: (a) aspectos clínicos (situación socioeconómica, datos antropométricos, comorbilidades, tabaquismo, clínica respiratoria, exacerbaciones, calidad de vida, escala ansiedad-depresión, actividades de la vida diaria, tratamientos); (b) función respiratoria (espirometría, gasometría arterial, hiperinsuflación, difusión, presiones respiratorias); (c) índice BODE (variable principal del estudio); (d) función muscular periférica, y (e) analítica sanguínea (incluida IgE y factores de riesgo cardiovascular). Además, se creará una seroteca para la futura determinación de biomarcadores. Los datos de los pacientes son anonimizados en una base de datos con un acceso jerárquico para garantizar la seguridad en los accesos a la información. El estudio CHAIN aportará información sobre la progresión de la EPOC y establecerá una red de investigadores para futuros proyectos relacionados con la enfermedad.
Studies that try to assess the natural evolution of chronic diseases are complex because they require multiple evaluations, multidisciplinary teams, and long follow-up periods. These difficulties increase in chronic obstructive pulmonary disease (COPD) due to its heterogeneity and the important loss of patients during follow-up given the high morbidity and mortality of this disease. For this reason, the study of the natural history of COPD constitutes a research priority for this disease and is a current challenge.1,2
One of the first studies published about the natural history of airflow limitation was written by Fletcher and Peto, which has been a reference document for pulmonologists.3 In this study, the population analyzed included only men and an 8-year follow-up period in the 1960s, a time when spirometry had not been well standardized and modern drugs, which have been shown to be able to influence the disease evolution, were not available. More recent studies that include women and have longer follow-up periods have shown important differences in the susceptibility to damage caused by tobacco use relating to the decline in FEV1.4,5
Currently, other cohort studies are underway that, with a multidimensional and longitudinal perspective, aim to study several aspects of the disease. Among these studies are the ECLIPSE6 and PAC-COPD7 cohorts, which aim to provide a possible phenotypic characterization of the disease; the BODE8 cohort, developing the multidimensional assessment of the disease; the cohort of the COPDGene9 study, aimed at studying genetic determinants of the disease; the Hokkaido cohort in Japan,10 which studies the characterizations of emphysema; or the SAPALDIA 1 and 211,12 studies in Switzerland that evaluate the role of environmental pollution and the differences between the sexes in FEV1 decline.
These described studies provide very relevant information, but their limitations include objectives that evaluate specific aspects of the disease instead of its entire multidimensional complexity, relatively short follow-up periods for a chronic disease such as COPD, and occasionally having small sample sizes or frequently taking FEV1 as the main evolutive parameter. In this sense, we know that FEV1, while still being a good prognostic parameter, has been shown to be insufficient to evaluate the phenotypic heterogeneity of COPD and multidimensional measurements have been demonstrated to be superior for evaluating patient prognosis.8
Given this situation, new longitudinal observational studies are necessary in order to understand the natural evolution of the disease from a multidimensional standpoint and according to clinical phenotypes.13 With this objective, the CHAIN (Chronic obstructive pulmonary disease History Assessment In SpaiN) study was designed as a national multi-center study with a cohort of smoker patients with COPD and smokers without COPD (control group). This article reports the method and the organization of the CHAIN study.
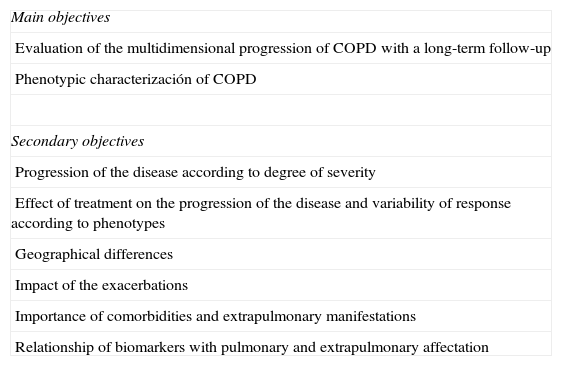
MethodCHAIN (clinicaltrials.gov NCT01122758) is a multi-center, observational study of prospective cohorts done in Spanish hospitals, including 2 cohorts: (a) a group of active smoker or ex-smoker patients with COPD (COPD cohort) and (b) a group of active smoker or ex-smoker patients without COPD (control cohort). Both cohorts will be followed-up for a period of at least 5 years, with complete check-ups every 12 months and telephone follow-ups every 6 months in order to assess the number of exacerbations and the vital state of the subjects. The project is included within the COPD integrated research programs (IRP) framework of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), and its objectives are summarized in Table 1.
Main and Secondary Objectives of CHAIN.
| Main objectives |
| Evaluation of the multidimensional progression of COPD with a long-term follow-up |
| Phenotypic characterización of COPD |
| Secondary objectives |
| Progression of the disease according to degree of severity |
| Effect of treatment on the progression of the disease and variability of response according to phenotypes |
| Geographical differences |
| Impact of the exacerbations |
| Importance of comorbidities and extrapulmonary manifestations |
| Relationship of biomarkers with pulmonary and extrapulmonary affectation |
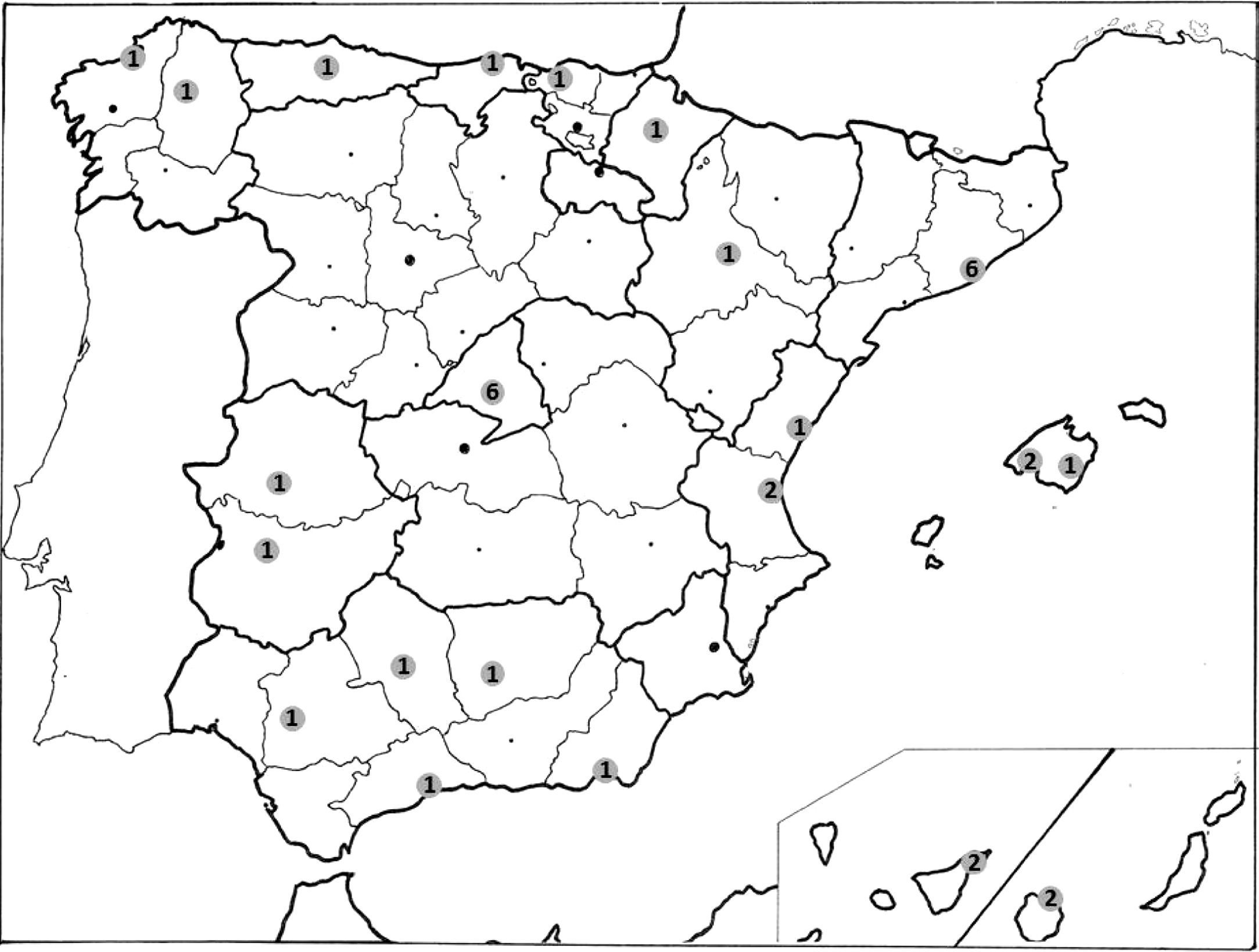
From the Directive Committee of the SEPAR COPD IRP, members of the COPD research area were contacted in order to propose the study to them. An initial meeting took place in April 2009, where the objectives of the project were specified and the scientific committee for the project was selected. During the following months, the scientific committee worked on the design of an initial draft of the study. Later, pulmonologists with research experience in COPD or those who had shown interest in participating were invited to a meeting before the start of the recruitment period in order to evaluate their participation in the project and to reach a consensus on the study protocol. This meeting took place in November 2009. At these meetings, it was decided that there would be a main researcher for every participating unit. After an initial contact with 52 hospitals, 36 centers from 13 Spanish regions decided to participate in the study (Fig. 1). Later, once the recruitment of cases had been started, a third meeting was held in April 2010 to evaluate the project start-up and to assess possible problems after patient recruitment.
Patient SelectionDue to the multidimensional nature of the project, the BODE index8 was established as the main variable of the study and basis for calculating the sample size. Contemplating an alpha error of 5% and a power of 80%, for the comparison of the progression on the BODE index between 2 samples of COPD participants (severe versus very severe COPD, or mild versus moderate COPD), since in the reference group the BODE is 0 by definition, and considering a mean BODE of 3 and standard deviation of 2,10 at least 300 participants are required per group in order to detect an increase of 0.6 units or higher in the BODE index between groups. Due to the number of participating centers, the reference was determined to be 50 cases and 10 controls per center.
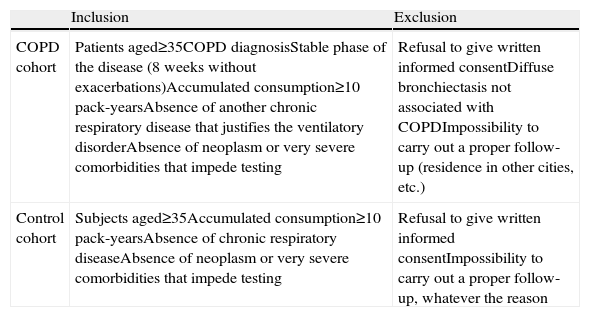
The inclusion and exclusion criteria for each study group are defined in Table 2. The patients were recruited consecutively from the outpatient consultations during scheduled appointments. The patients were in stable clinical situation and had been free from exacerbations for at least 8 weeks. It was decided that the selected cases and controls should be paired for age and sex.
Inclusion and Exclusion Criteria.
| Inclusion | Exclusion | |
| COPD cohort | Patients aged≥35COPD diagnosisStable phase of the disease (8 weeks without exacerbations)Accumulated consumption≥10 pack-yearsAbsence of another chronic respiratory disease that justifies the ventilatory disorderAbsence of neoplasm or very severe comorbidities that impede testing | Refusal to give written informed consentDiffuse bronchiectasis not associated with COPDImpossibility to carry out a proper follow-up (residence in other cities, etc.) |
| Control cohort | Subjects aged≥35Accumulated consumption≥10 pack-yearsAbsence of chronic respiratory diseaseAbsence of neoplasm or very severe comorbidities that impede testing | Refusal to give written informed consentImpossibility to carry out a proper follow-up, whatever the reason |
The variables collected during the follow-up were agreed upon by the researchers. Due to the fact that the objective was to carry out a multidimensional evaluation of COPD, the number of variables had to be high. During the development of the method, some variables were excluded to make the protocol simpler and more viable, but the researchers were given the opportunity to register other variables in case a group of researchers wished to carry out a sub-study including these data. After several consensus sessions, the selected variables were grouped according to the following aspects: socioeconomic data, comorbidities, smoking, clinical data, treatments, and complementary studies.
The socioeconomic data that were registered included: marital state, level of education, occupation, employment, and social class. Employment status was collected in an open field. In addition to the comorbidities included in the Charlson index,14 an extensive questionnaire was established for the systematic compilation of other comorbidities. In the case of depression and anxiety, these were evaluated with the Hospital Anxiety and Depression Scale (HADS).15
As for smoking, patients are registered as either active smoker or ex-smoker, as well as data for age of initiation and calculated accumulated consumption in pack-years. In addition, at each visit, co-oximetry is done in the exhaled air to verify if there have been any changes in tobacco habit. The co-oximetries are all done with the same device (PiCO Smokerlyzer, Bedfont Scientific, Kent, United Kingdom) acquired specifically for this project.
The clinical data obtained cover the following aspects: the date of diagnosis, the presence of respiratory symptoms (dyspnea, cough, expectoration, and color of sputum), symptoms related with sleep (frequent snoring, insomnia), and cardiologic symptoms (presence of malleolar and orthopneic edemas). Dyspnea is quantified by means of the modified Medical Research Council (MRC) scale.16 The impact of the symptoms is assessed by means of the Clinical COPD Questionnaire (CCQ).17 In addition, activities of daily living are evaluated with the London Chest Activity of Daily Living (LCADL) scale.18 The variables for exacerbations in the previous year are registered as part of the protocol (Table 3), identifying if the information were revealed by the patient or instead obtained from the patient files. Quality of life is evaluated by mean of the COPD Assessment Test (CAT) questionnaire.19
Variables Registered Related With Annual Exacerbations.
| Number of unscheduled visits to the physician due to COPD |
| Number of visits to the primary care center emergency services |
| Number of visits to hospital emergency room |
| Number of hospitalizations: ward or ICU |
| Number of episodes requiring non-invasive ventilation |
| Number of episodes requiring invasive ventilation |
| Number of cycles of antibiotics due to exacerbations |
| Numerous of cycles of systemic corticosteroids |
| Date of the latest exacerbation |
As for treatment, the drugs compiled are shown in Table 4. It also registers anti-flu and anti-pneumonia vaccinations, home therapies such as oxygen therapy and home mechanical ventilation, as well as any type of respiratory rehabilitation program. The patients of the COPD cohort are treated according to the current national and international recommendations for the treatment of COPD, as decided by the head researcher. All the included subjects who are active smokers received minimal anti-smoking intervention, trying to advance in the process of quitting according to the Prochaska and DiClemente method20 and offering complete anti-smoking treatment according to national guidelines.21
Pharmacological Groups.
| Bronchodilators |
| Inhaled and oral corticosteroids |
| Methylxanthines |
| Mucolytics/antioxidants |
| Anti-smoking drugs |
| Diuretics |
| Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists |
| Beta-blockers |
| Blood thinners−anticoagulants |
| Anxiolytics-antidepressants |
| Statins |
| Oral anti-diabetic medication−insulin |
| Substitutive hormone therapy |
The complementary studies that were ordered are listed in Table 5. All the analyses are done in the reference laboratories of each center. Blood samples were taken for hemogram and general biochemistry (with special interest in cardiovascular risk parameters, total IgE), and the first analysis included determination of α1-antitripsin levels. Finally, 35-ml EDTA tubes were extracted to centrifuge (3000rpm for 10min at room temperature) and extract serum, which was stored at −80°C for later biomarker studies. For storage, at least 6 aliquots of 500μl were separated before freezing. Each center was provided with labels identifying each aliquot by center code, case number, and aliquot number.
Complementary Tests Ordered at the Annual Check-Ups of the COPD Cohort.
| Analysis |
| Hemogram |
| General biochemistry: glucose, creatinine, urea, albumin, lipid profile |
| Fibrinogen |
| Alpha-1-antitrypsin |
| C-reactive protein |
| Total IgE |
| Glycated hemoglobin |
| Sample for serum bank |
| Simple chest radiography |
| CO-oximetry |
| Nutritional assessment |
| Body mass index |
| Waist and hip perimeter |
| Bioimpedance |
| Lung function tests |
| Spirometry with bronchodilator test |
| Lung volumes |
| Diffusion tests |
| Arterial blood gases |
| Muscle function tests |
| Maximal inspiratory and expiratory pressures |
| Muscle strength of the upper extremities |
| 6-Min walk test |
For the nutritional assessment, the body mass index was calculated and a body bioimpedance study was done. All the bioimpedance studies were carried out with the same device, an Omron BF500 (Omron Corporation, Kyoto, Japan) acquired specifically for this project. With this technique, body and visceral fat mass can be estimated. Lung function tests were done with the equipment available in each participating hospital, registering the absolute values of each determination to be able to apply the same theoretical values for the analysis of the results. The peripheral muscle strength study was done with a GRIP-A hand dynamometer (Takei Scientific Instruments, Niigata, Japan), acquired specifically for this project. The 6-min walk test22 was done in accordance with international recommendations.23 If the patient did not know the test, a second test was done (separated by at least 30min of rest) to make amends for the possible training effect.
At each annual and semestral office visit, the vital state of the subject will be registered and, in case of death, the cause of death will also be reported (coded as: respiratory, cardiovascular, cerebrovascular, lung neoplasm, other neoplasms, sudden death, other or unknown) as well as the exacerbation variables (Table 3). Specifying the cause of death in each case will be the responsibility of the local researcher, taking into account the information provided by family members and the patient files.
Notebooks will be provided to collect the following exacerbation variables: the time of the exacerbation in days, the main location of care (home, outpatient, hospital emergency department, hospitalization or ICU), the Anthonisen criteria24 and the treatment received.
For the control cohort, the same data were collected as in the case cohort, but the respiratory function tests are simplified, requiring at least spirometry and an oxygen saturation measurement by pulse-oximetry.
Follow-UpThe case recruitment period lasted 26 months, from January 15, 2010 to March 31, 2012. After the end of the inclusion period, there were a total of 991 cases and 132 controls. The follow-up of the subjects includes annual office visits, where all the information will be collected for each case. In addition, every 6 months a telephone call is programmed to compile data about the number of exacerbations and to verify the vital status of the subject. Finally, the patients were instructed that they could come to the consultation any time if they were having an exacerbation with the aim to collect data about the exacerbations. The project includes a scientific notebook (SNB) to collect data from the annual scheduled visits and the biannual phone calls.
A company was selected with experience in the design of web medical applications (Claveweb, Seville, Spain) to create the project webpage (www.chain.org.es). The web page offers information about the project and provides the researchers and scientific committee access to the data. The web has a hierarchical structure, so that the scientific committee has access to all the information, but the local researchers can only access the data from their respective centers. The web application also has an option to download data for the scientific committee in order to be able to download data and do quality controls.
A data manager was hired to communicate with the researchers and to resolve incidences, maintain the database, and control the quality of the data. The quality control entails confirming the veracity of the registered data. The quality controls of the procedures carried out at each center are done by each participating hospital, following the internal regulations at their centers. The project manager reviews the database weekly to identify lost, inconsistent or extreme values. In these cases, the project manager contacts the researcher to confirm or correct the final value. In addition, the project manager periodically sends the researchers an e-mail with the recruitment information of cases and the state of the project.
EthicsThe present protocol was approved by the Healthcare Research Ethics Committees of the participating centers, and the principles of the Declaration of Helsinki are observed for research with human subjects. All the participants were informed about the nature of the study and its objectives, and they signed their consent to participate, while being able to withdraw this consent at any time.
All the procedures and variables related with this paper are compiled in a SNB. There is an SNB for each included subject, regardless of the group to which he/she belonged, and in said SNB all the variables related with the study are annotated.
During the study, no personal data of the participating patients are taken which would enable the patient to be identified. These data were made anonymous in the web application. The data obtained will be maintained under strict confidentiality (Law 15/1999, protection of personal data) and only the researcher responsible for the project in each center will have access to them.
The biological samples related with the study, as well as the SNB, are coded in order to guarantee the confidentiality of the sample and the data. During the study period, the SNB are stored under lock and key, which is kept exclusively by the researcher in charge of the project at the center and, after their transcription to the database, will be destroyed.
StatisticsThe results for each variable will be presented by means, standard deviation, minimum, median, maximum, and number of cases validated in the case of the continuous variables, and using the case count for each category and the frequency related to the total number of responses in of the categorical variables will be done. For the statistical comparisons between groups, in the continuous variables an initial ANOVA will be done and then later a bilateral Student's t test or a χ2 for the categorical variables. The Spearman's test will be used to determine correlation between non-parametric variables, and Pearson's for the parametrical ones. In all the statistical tests done, a level of statistical significance of 0.05 will be used.
DiscussionThis present study intends to provide a detailed vision of the method used, the organization of the paper and the objectives that were posed in the CHAIN study, as well to show the steps taken to overcome the complexity of an ambitious study with a considerable number of participating centers and researchers following an extensive protocol. CHAIN has great potential to contribute to our understanding of COPD by providing data about the characteristics of the disease and the evolution of each variable studied over time, both in an isolated manner as well as in conjunction. This will be able to not only describe the long-term behavior of the disease, but also establish relationships between these clinical behaviors and biological markers of the patients included.
CHAIN intends to fill a gap that has not been sufficiently dealt with by other cohort studies that are under way. In spite of the acknowledgement of COPD's multidimensional complexity, few papers have approached the evolution of this disease over time with the objective of evaluating its natural history from a multidimensional viewpoint.
After finalizing the recruitment of centers and the establishment of the complete study protocol, 36 centers have been fully included in the project with the inclusion of cases during the recruitment period. Some centers that were interested in the project were not able to initiate the study: some due to local organizational problems, others due to the extensive amount of data to be registered, or occasionally due to renovations, relocation of the center or not having research staff dedicated to the project.
The variables to be collected were debated intensely. During the months of the scientific committee's work prior to the start of the study, the meetings of November 2009 and April 2010 and the many exchanges of opinions between the scientific committee and the participating hospitals, the study protocol improved bit by bit by including some variables, ruling out others, and modifying some that had already been selected. Some of the proposals were very interesting but had to be ruled out in order to make the protocol accessible to the majority of centers, or instead due to cost limitations. Some of these examples are the registration of respiratory disorders during sleep, CT scans, induced sputum studies, bronchiectasis studies or complementary studies in order to objectively evaluate cardiovascular comorbidity. These aspects were not included in the study, but they were kept as an idea for sub-studies among researchers who were interested. Thus, CHAIN plans to incorporate sub-studies that, while maintaining the essential core of the study, will be able to delve into specific aspects of COPD.
One of the key aspects for the success of this type of projects is proper programming of the webpage database and the work of the data manager, who should be on top of the project, constantly supervising the quality of the information received and encouraging researchers that fall behind. Specifically, establishing a periodical informational bulletin contributes to the awareness of the researchers for the inclusion of cases.
Because of the differing amounts of time that the ethics committees at the various centers are required to approve the protocol, there was great variability in the start date at the different centers. Some centers gave their approval more quickly, others took several weeks, and in some cases a fee was required in order to assess the project.
The limitations of the project include, first of all, the impossibility to include all the possible variables. As we have commented, some had to be excluded at the meetings in order to make the protocol viable. Although exclusion criteria were not defined related to the characteristics of the disease, some phenotypes, such as mixed COPD-asthma, currently being debated, are not included in detail according to current diagnostic criteria25 because the diagnostic criteria had not been published when the study was initiated. The second limitation is the complexity of the protocol for collecting clinical information. COPD is a complex multidimensional disease and, despite the fact that the scientific committee at its several meetings tried to make the protocol as simple as possible, the enormous variability of the clinical presentation of this disease means that collecting the information is similarly complex in order to have the most complete information possible at our disposal. The selection of centers and researchers was not random, but instead depended on their willingness, which may limit representativity and the extrapolation of results. The magnitude of the likely loss of participants to follow-up should be monitored.
In short, CHAIN is an observational study of prospective cohorts, designed with the objective to make a long-term assessment of the natural history of the disease from a multidimensional standpoint. Patient characteristics will provide relevant information about the progression of COPD and will establish the foundation for the phenotypic stratification of the patients, which should allow us to advance in our knowledge of this disease. Finally, we believe that this study will help to create a solid structure of Spanish national researchers in the field of COPD who will promote the development of future projects with similar standards for excellence.
FundingCHAIN has been financed in its first 3 years by AstraZeneca under the existing clauses in the contract signed with the Respira Foundation (Spanish Lung Foundation).
Conflict of InterestsThe authors declare having no conflicts of interests.
The authors of this present paper would like to thank AstraZeneca for funding the project and providing logistic support for the meetings. Likewise, the authors would like to acknowledge the researchers who participated in the project, without whom this study would never have been possible.
Scientific committee: Ciro Casanova Macario (coordinador), Pilar de Lucas, Juan Pablo de Torres, José Luis Lopez-Campos, José María Marín, German Peces-Barba, Juan José Soler Cataluña, Joan B Soriano.
Local researchers:
ANDALUCÍA. José Calvo Bonachera, Hospital de Torrecárdenas, Almería. Nuria Feu Collado, Hospital Universitario Reina Sofía, Córdoba. Celia Lacárcel Bautista, Hospital Ciudad de Jaén, Jaén. Adolfo Domenech, Hospital Universitario Carlos Haya, Málaga. Inmaculada Alfageme Michavila, Hospital Universitario de Valme, Sevilla.
ARAGÓN. José María Marín Trigo, Hospital Universitario Miguel Servet, Zaragoza.
ASTURIAS. Cristina Martínez González, Hospital Central de Asturias, Oviedo.
BALEARES. Rosa Irigaray, Hospital de Manacor, Manacor. Borja García-Cosío Piqueras, Hospital Son Espases, Mallorca. Isabel Mir Viladrich, Hospital Son Llátzer, Mallorca.
CANARIAS. Carlos Cabrera López, Hospital Dr. Negrín, Las Palmas de Gran Canaria. Alejandro Sánchez Acosta, Hospital Insular de Las Palmas, Las Palmas de Gran Canaria. Ciro Casanova Macario, Hospital Universitario de la Candelaria, Santa Cruz de Tenerife. Juan Abreu González, Hospital Universitario de Canarias, Santa Cruz de Tenerife.
CANTABRIA. Ramón Agüero Balbin, Hospital Marqués de Valdecillas, Santander.
CATALUÑA. Eva Balcells, Hospital del Mar, Barcelona. Elena Miguel Campos, Hospital Sant Joan Despí, Barcelona. Alicia Marin, Hospital German Trias y Pujol, Badalona, Barcelona. Ingrid Solanes García, Hospital San Pablo y la Santa Cruz, Barcelona. Antonia Llunel Casanova, Hospital de Tarrasa, Tarrasa. Amalia Moreno, Hospital Parc Taulí, Sabadel.
EXTREMADURA. Francisca Lourdes Márquez Pérez, Hospital Infanta Cristina, Badajoz. Juan Antonio Riesco Miranda, Hospital San Pedro Alcántara, Cáceres.
GALICIA. Julia Tabara Rodríguez, Hospital Juan Canalejo, La Coruña. Rafael Golpe Gómez, Hospital General Calde, Lugo.
MADRID. Germán Peces-Barba Romero, Fundación Jiménez Díaz, Madrid. Miriam Calle Rubio, Hospital Clínico San Carlos, Madrid. Javier de Miguel Díez, Hospital Gregorio Marañón, Madrid. Pilar de Lucas Ramos, Hospital Gregorio Marañón, Madrid. Francisco García Río, Hospital La Paz, Madrid. Salvador Díaz Lobato, Hospital Ramón y Cajal, Madrid.
NAVARRA. Juan Pablo de Torres Tajes, Clínica Universitaria de Navarra, Pamplona.
PAÍS VASCO. Juan Bautista Galdiz Iturri, Hospital de Cruces, Bilbao.
VALENCIA. Margarita Marín Royo, Hospital General de Castellón, Castellón. Juan José Soler Cataluña, Hospital General de Requena, Requena. Alfredo de Diego Damia, Hospital Universitario La Fe, Valencia.
Data manager: Domingo León Mora, Hospital Universitario de la Candelaria, Santa Cruz de Tenerife.
Appendix 1 contains the list of the group of researchers (CHAIN).
Please cite this article as: López-Campos JL, et al. Chronic obstructive pulmonary disease History Assessment in Spain: una valoración multidimensional de la enfermedad pulmonar obstructiva crónica. Método y organización del trabajo. Arch Bronconeumol. 2012;48:453–9.