Non-invasive ventilation (NIV) is the standard treatment for respiratory impairment in patients with amyotrophic lateral sclerosis (ALS).1 In a 2006 study, Bourke et al.2 showed that NIV improved quality of life and survival in patients with ALS. However, more recent studies have found that higher compliance is associated with improved survival,3,4 highlighting the importance of achieving good compliance, particularly in the light of recent findings emphasizing the need for early NIV initiation.5,6 However, NIV compliance changes over time are not well understood. Jackson et al.7 reported a progressive increase in compliance in an ALS cohort; however, the small number of patients and the study design did not allow the authors to draw conclusions about changes in compliance over time.
The aim of our study was to assess changes in NIV compliance during the first year after early initiation of the treatment.
This post-hoc retrospective study analyzed patients from a previously published study.8 All participants were patients of the ALS center in Bordeaux, who were treated with NIV between September 2017 and June 2021. The indication for NIV was based on current guidelines.1
NIV was initiated at home by a team of specialized physiotherapists from the Bordeaux University Hospital. The initiation period was 30 days, after which patients were reassessed every 3 months. At each assessment, parameters were adjusted according to patients’ needs (e.g., a feeling of insufficient air), monitoring results (presence of leaks, elevated apnea-hypopnea index [AHI]), or residual hypoventilation according to partial pressure of carbon dioxide (PaCO2) or oximetry data. The goal was to achieve respiratory comfort, AHI <10, leak rate <24L/min, respiratory rate <20cycles/min, target volume of 8–10mL/kg, and nocturnal SpO2 correction (nocturnal SpO2 <90%, time <5%) with PaCO2 <6kPa.
Data were collected from the initiation of home NIV through month 12 using a telemonitoring platform (Airview; ResMed Ltd., Sydney, Australia). NIV compliance was determined based on the average daily use during the last 30 days.
Patients were divided into three groups according to their compliance (insufficient use, <5h; night-time use, 5–8h; daytime and night-time use, >8h) at 1 month (M1), 4 months (M4), and 12 months (M12) after NIV initiation. The participants were further classified into three subgroups according to NIV compliance over time (stable, increased use, and decreased use) between M1 and M4 and between M4 and M12.
Patients who died before M4 were not included in the analysis of NIV compliance between M1 and M4. Similarly, patients who died between M4 and M12 were not included in the analysis of NIV compliance between M4 and M12. Patients who died or were lost to follow-up before M1 were not included in the analysis, and those lost to follow-up after M1 were considered deceased and not included in the analysis.
The primary objective was to access changes in compliance between M1 and M4 and M4 and M12. The secondary objective was to identify factors associated with changes in compliance over time.
Data are expressed as mean±standard deviation (SD) for continuous variables and as relative frequencies for categorical variables. Differences between continuous variables were assessed using the Kruskal–Wallis test, and the chi-square test was used for categorical variables. Statistical analyses were performed using RStudio version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
All the patients gave their informed consent to participate in the study, and it was approved by the Research Ethics Committee of the University Hospital of Bordeaux (reference: CER-BDX 2024-158).
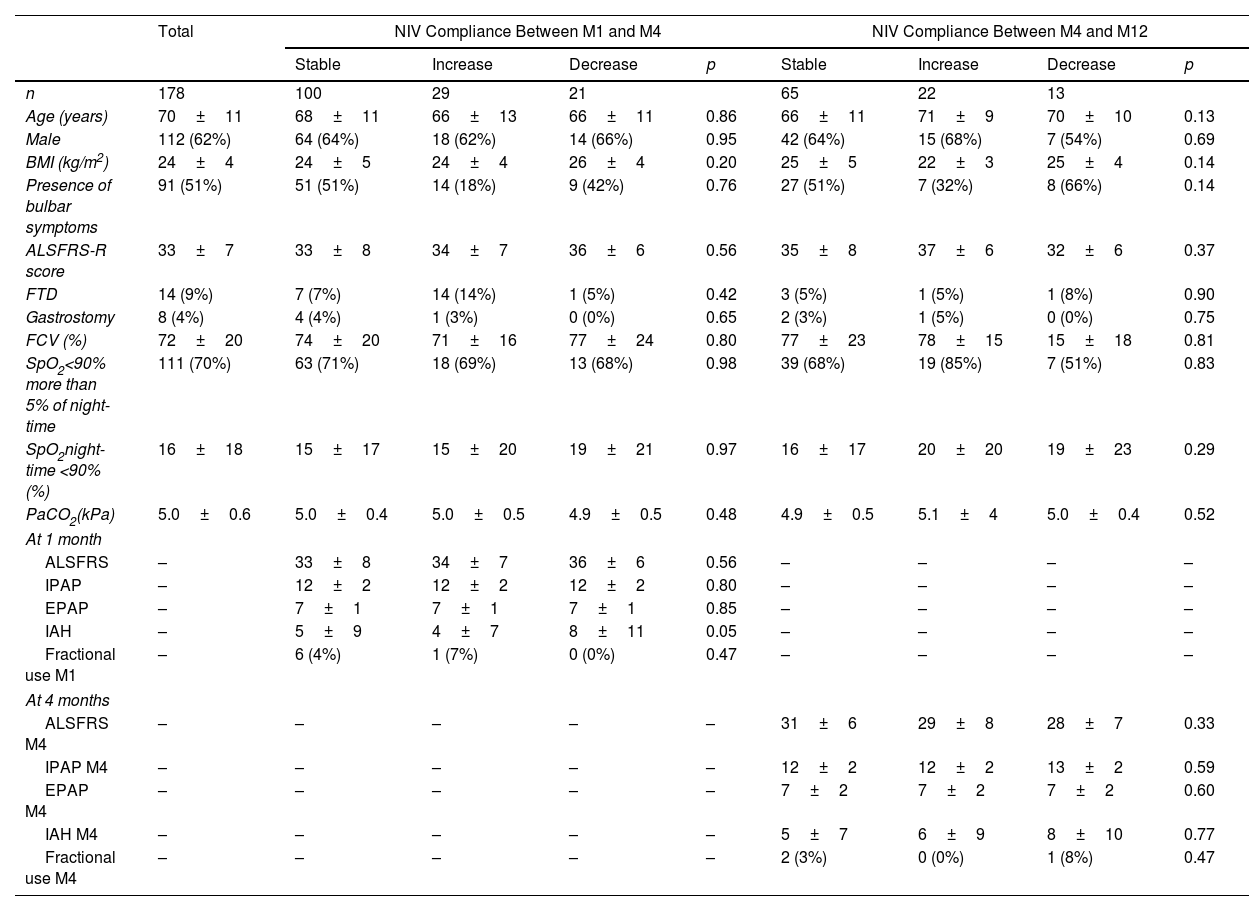
Home-based NIV was initiated in 184 patients, of whom 178 were included in the analysis (3 died and 3 were lost to follow-up before M1). The participants were predominantly male (62%), with a mean age of 70±11 years and a mean ALS Functional Rating Scale-Revised (ALSFR) score of 33±7 (Table 1).
Patients Characteristics According to Non-invasive Ventilation Compliance Over Time.
| Total | NIV Compliance Between M1 and M4 | NIV Compliance Between M4 and M12 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Stable | Increase | Decrease | p | Stable | Increase | Decrease | p | ||
| n | 178 | 100 | 29 | 21 | 65 | 22 | 13 | ||
| Age (years) | 70±11 | 68±11 | 66±13 | 66±11 | 0.86 | 66±11 | 71±9 | 70±10 | 0.13 |
| Male | 112 (62%) | 64 (64%) | 18 (62%) | 14 (66%) | 0.95 | 42 (64%) | 15 (68%) | 7 (54%) | 0.69 |
| BMI (kg/m2) | 24±4 | 24±5 | 24±4 | 26±4 | 0.20 | 25±5 | 22±3 | 25±4 | 0.14 |
| Presence of bulbar symptoms | 91 (51%) | 51 (51%) | 14 (18%) | 9 (42%) | 0.76 | 27 (51%) | 7 (32%) | 8 (66%) | 0.14 |
| ALSFRS-R score | 33±7 | 33±8 | 34±7 | 36±6 | 0.56 | 35±8 | 37±6 | 32±6 | 0.37 |
| FTD | 14 (9%) | 7 (7%) | 14 (14%) | 1 (5%) | 0.42 | 3 (5%) | 1 (5%) | 1 (8%) | 0.90 |
| Gastrostomy | 8 (4%) | 4 (4%) | 1 (3%) | 0 (0%) | 0.65 | 2 (3%) | 1 (5%) | 0 (0%) | 0.75 |
| FCV (%) | 72±20 | 74±20 | 71±16 | 77±24 | 0.80 | 77±23 | 78±15 | 15±18 | 0.81 |
| SpO2<90% more than 5% of night-time | 111 (70%) | 63 (71%) | 18 (69%) | 13 (68%) | 0.98 | 39 (68%) | 19 (85%) | 7 (51%) | 0.83 |
| SpO2night-time <90% (%) | 16±18 | 15±17 | 15±20 | 19±21 | 0.97 | 16±17 | 20±20 | 19±23 | 0.29 |
| PaCO2(kPa) | 5.0±0.6 | 5.0±0.4 | 5.0±0.5 | 4.9±0.5 | 0.48 | 4.9±0.5 | 5.1±4 | 5.0±0.4 | 0.52 |
| At 1 month | |||||||||
| ALSFRS | – | 33±8 | 34±7 | 36±6 | 0.56 | – | – | – | – |
| IPAP | – | 12±2 | 12±2 | 12±2 | 0.80 | – | – | – | – |
| EPAP | – | 7±1 | 7±1 | 7±1 | 0.85 | – | – | – | – |
| IAH | – | 5±9 | 4±7 | 8±11 | 0.05 | – | – | – | – |
| Fractional use M1 | – | 6 (4%) | 1 (7%) | 0 (0%) | 0.47 | – | – | – | – |
| At 4 months | |||||||||
| ALSFRS M4 | – | – | – | – | – | 31±6 | 29±8 | 28±7 | 0.33 |
| IPAP M4 | – | – | – | – | – | 12±2 | 12±2 | 13±2 | 0.59 |
| EPAP M4 | – | – | – | – | – | 7±2 | 7±2 | 7±2 | 0.60 |
| IAH M4 | – | – | – | – | – | 5±7 | 6±9 | 8±10 | 0.77 |
| Fractional use M4 | – | – | – | – | – | 2 (3%) | 0 (0%) | 1 (8%) | 0.47 |
Data are expressed as mean±SD or n (%) unless otherwise stated. ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; BMI: body mass index; EPAP: expiratory positive airway pressure; FTD: frontotemporal dementia; FVC: forced vital capacity; IAH: intra-abdominal hypertension; IPAP: inspiratory positive airway pressure; NIV: non-invasive ventilation; PaCO2: arterial carbon dioxide tension; SpO2: oxygen saturation measured by pulse oximetry.
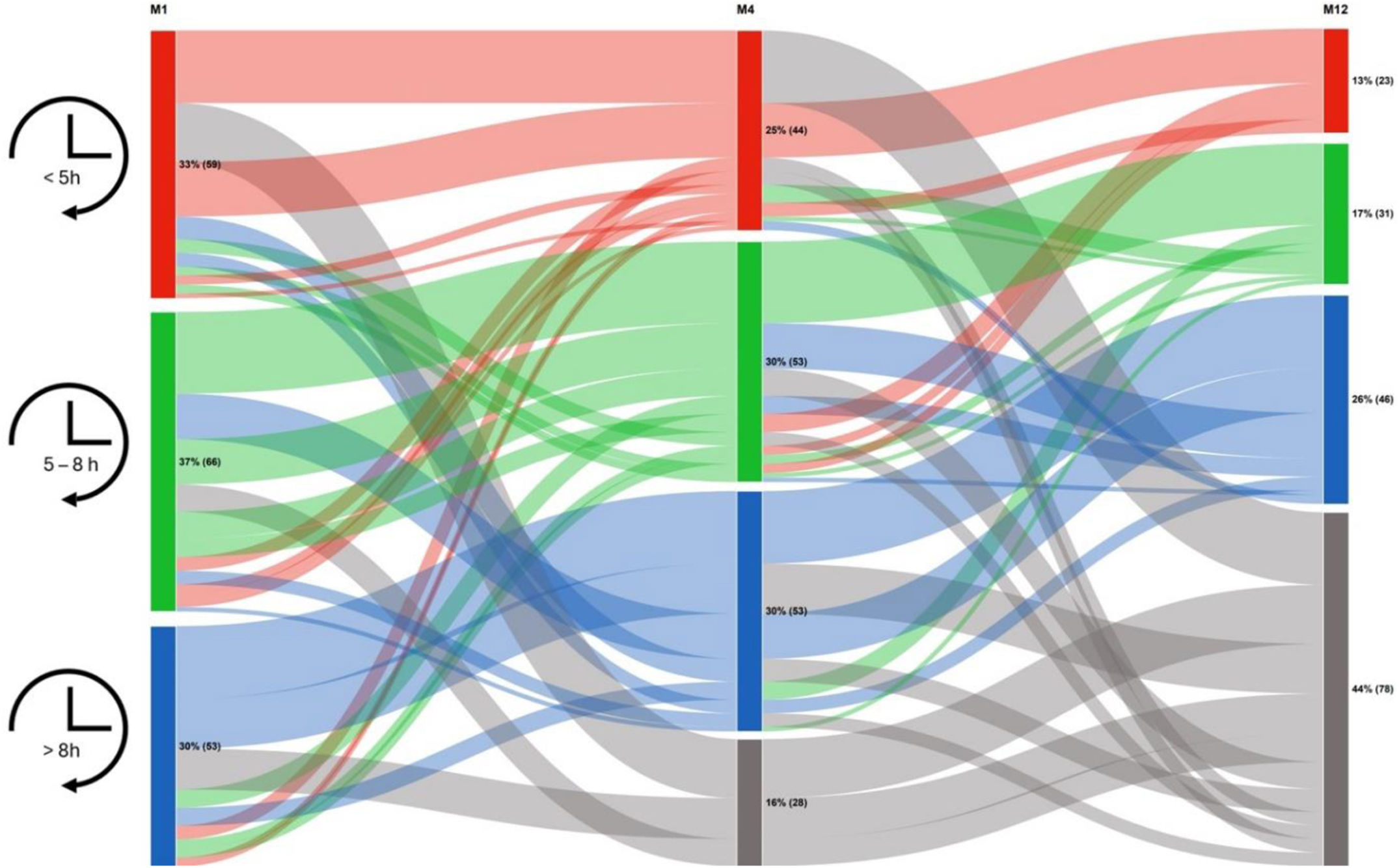
Fig. 1 shows changes in compliance over time. In total, 28 (16%) patients died before M4, and 78 (44%) had died by M12. The percentage of survivors in the compliance groups (<5, 5–8, and >8h) did not differ over time (33%, 37%, and 30% at M1; 29%, 35%, and 35% at M4; and 23%, 31%, and 46% at M12, respectively; p=0.11).
The analysis of compliance over time revealed that between M1 and M4, compliance was stable in 100 (67%) patients, increased in 29 (19%) patients, and decreased in 21 (14%) patients. Between M4 and M12, compliance was stable in 65 (65%) patients, increased in 22 (22%) patients, and decreased in 13 (13%) patients. No factors were associated with changes in compliance (Table 1).
In total, between M1 and M12, 55 (55%) patients showed stable compliance, 30 (30%) showed an increase, and 15 (15%) showed a decrease.
Our study assessed changes in NIV compliance over time in patients with ALS. Our main finding was that NIV compliance was stable over the 12 months following initiation in nearly two-thirds of the patients.
NIV compliance is a predictive factor for survival.4,5,8 However, because ALS is a neurodegenerative disease characterized by progressive worsening of muscle damage and respiratory impairment,9–11 dependence on NIV treatment is likely to increase as the severity of respiratory impairment increases. Alternatively, because NIV is a palliative treatment for symptomatic ALS,12 some patients may stop treatment because it no longer provides comfort or prolongs a situation they find unbearable.13 In a small study of ALS patients, Jackson et al.7 found that NIV treatment compliance increased during the first year, with 53% of patients showing good compliance at 1 month and 68% at 1 year. However, only 18 patients remained in the study after 1 year, and no data were available on individual compliance trends.7 In our study, the proportion of patients with poor compliance was lower than that reported by Jackson et al. (33% vs. 48% at 1 month and 23% vs. 33% at 12 months, respectively), and compliance remained stable after the first month of treatment in our patients. Furthermore, no factors, including the ALSFR score, were associated with changes in compliance over time. Our findings suggest that the trend towards increased compliance reported by Jackson et al. was due to the death of patients with poor compliance (as the authors hypothesized in their discussion). It is also important to note that this study addressed at home NIV initiation and may be not transposable to patient for whom initiation was made as inpatient. However, Volpato et al.14 showed no difference in NIV acceptance and adherence between in and outpatient's groups.
In our study, the mean pressure support was low, maybe partly due to a short at home titration. As a reminder, it is the first experiment to introduce home ventilation in France, and, as discussed in the source study,8 many adjustments still need to be made, notably titration, which is insufficient and too gradual. Even though it is legitimate to think this under-assistance can influence observance, there does not seem to be any difference between the groups, and we did not observe lower adherence than what has already been described in the literature.7
We do not have data regarding respiratory muscle assessment via the Peak Cough Flow, nor the number of patients under In/Exsufflator. This particular point is not well known nor studied in terms of its potential impact on NIV observance, and should be addressed in the future.
Other factors that can influence NIV observance and adherence are frontotemporal dementia, as these patients could be hard to manage when it comes to such therapy, and gastrostomy use. However, these factors do not appear to influence observance and adherence in our study.
The use of group classifications to assess patient compliance is debatable. We proposed clinically relevant cut-off points to measure compliance: 5h was based on a recent study showing that NIV use for >5h was significantly associated with improved survival,15 and >8h indicated nocturnal and daytime use.
Our finding that NIV compliance was stable over time in our ALS cohort highlights the need to achieve good compliance in the first month after NIV initiation. The first month of NIV treatment appears to be a decisive period for patient outcomes, which raises the issue of when16 and where to initiate treatment (as hospital inpatients, day hospital outpatients or at home8,16).
In summary, our assessment of NIV compliance over 1 year revealed that compliance did not change significantly after 1 month in most patients, highlighting the importance of achieving good compliance during the NIV initiation period.
Conflicts of InterestThe authors have not declared a specific grant for this particular research from any funding agency in the public, commercial or not-for-profit sectors.
Pierre Schilfarth reports personal fees from SOS Oxygène, outside the submitted work.
Leo Grassion reports grants and personal fees from AADAIRC, grants from FGLMR, personal fees from Vivisol, personal fees from ALMS, personal fees from Resmed, personal fees from ASV, personal fees from Asten, personal fees from SOS Oxygène, personal fees from Boerhinger Ingheleim, personal fees from Isis medical, and personal fees from Alizée santé, outside the submitted work.
No potential conflict of interest relevant to this article was reported for others authors.