Monitoring changes in symptoms over time during long-term nocturnal home non-invasive ventilation (NIV) using patient-reported outcome measures is crucial. This study aimed to identify factors associated with changes in the S3-NIV total score, its two domains (“respiratory symptoms” and “sleep and NIV-related side effects”) and individual item responses.
MethodsWe conducted a retrospective, longitudinal data analysis of a cohort of adults with chronic respiratory failure treated with NIV. Data were obtained from a French homecare provider. Multivariate linear and multinomial ordinal mixed effect models were used to identify factors associated with changes in S3-NIV scores over time.
ResultsMedian follow-up was 2 years for 2135 participants. Each participant completed a median of five S3-NIV questionnaires; totaling 11,359 analyzed questionnaires. Type of respiratory condition, sex, age and time since NIV initiation were associated with change in S3-NIV score over time. NIV adherence was not associated with total S3-NIV score but high adherence was associated with more severe respiratory symptoms and an improvement in sleep and NIV-related side effects during the follow-up. Intensity of pressure support was associated with a lower total S3-NIV score and more side effects. Face masks and supplemental oxygen were associated with a lower S3-NIV total score.
ConclusionChanges in S3-NIV scores over time are associated with the individual's characteristics and NIV settings. Analysis of the two domains and individual items of the S3-NIV could increase understanding of the difficulties experienced by people on NIV.
Non-invasive home ventilation (NIV) is the most frequently used strategy for the management of chronic hypercapnic respiratory failure (CHRF).1,2 As well as improving gas exchange and reducing hospitalization and mortality,3–5 NIV has a major, positive impact on patient-reported outcomes, including health related quality of life (HRQoL)6 and sleep quality.7 Several health related quality of life instruments have been developed to evaluate health-related quality of life, including the Saint Georges Respiratory Questionnaire (SGRQ), the Maugeri Foundation questionnaire (MRF-26), and the Severe Respiratory Insufficiency questionnaire (SRI).5,6,8–16 The SRI questionnaire and the MRF-26 are commonly used in NIV trials to assess aspects of health-related quality of life; however, their length and complex scoring algorithms limit their use in everyday clinical practice. Furthermore, none of these questionnaires evaluate the treatment-related side effects of NIV.
The S3-NIV questionnaire, a specific patient-reported outcome measure (PROM) designed to monitor individuals treated with home NIV, is a short, reliable and easy-to-apply questionnaire.17 It measures respiratory symptoms, sleep quality and NIV-related side effects. English, French, Portuguese, and Danish translations are available.17–19 The S3-NIV has been validated against other known questionnaires.17 Moreover, S3-NIV scores have been reported for different disease groups.17,18 However, the full potential of the questionnaire to document individual clinical trajectories during follow-up of NIV treatment remains to be established. Guerreiro et al. established a minimal clinically important difference (MCID) using an anchor-based approach corresponding to hospitalizations for acute respiratory exacerbations.20 However, these clinical events represent major alterations in health status. When monitoring long-term NIV, it is necessary to assess less dramatic changes, such as the impact of a change in NIV settings, interface, additional humidification or initiation of supplemental oxygen.
Therefore, the objectives of this study were to investigate factors associated with the time-course of the scores of (1) the total S3-NIV, (2) its two domains, and (3) its 11 items in a large cohort of individuals treated with long-term home nocturnal NIV.
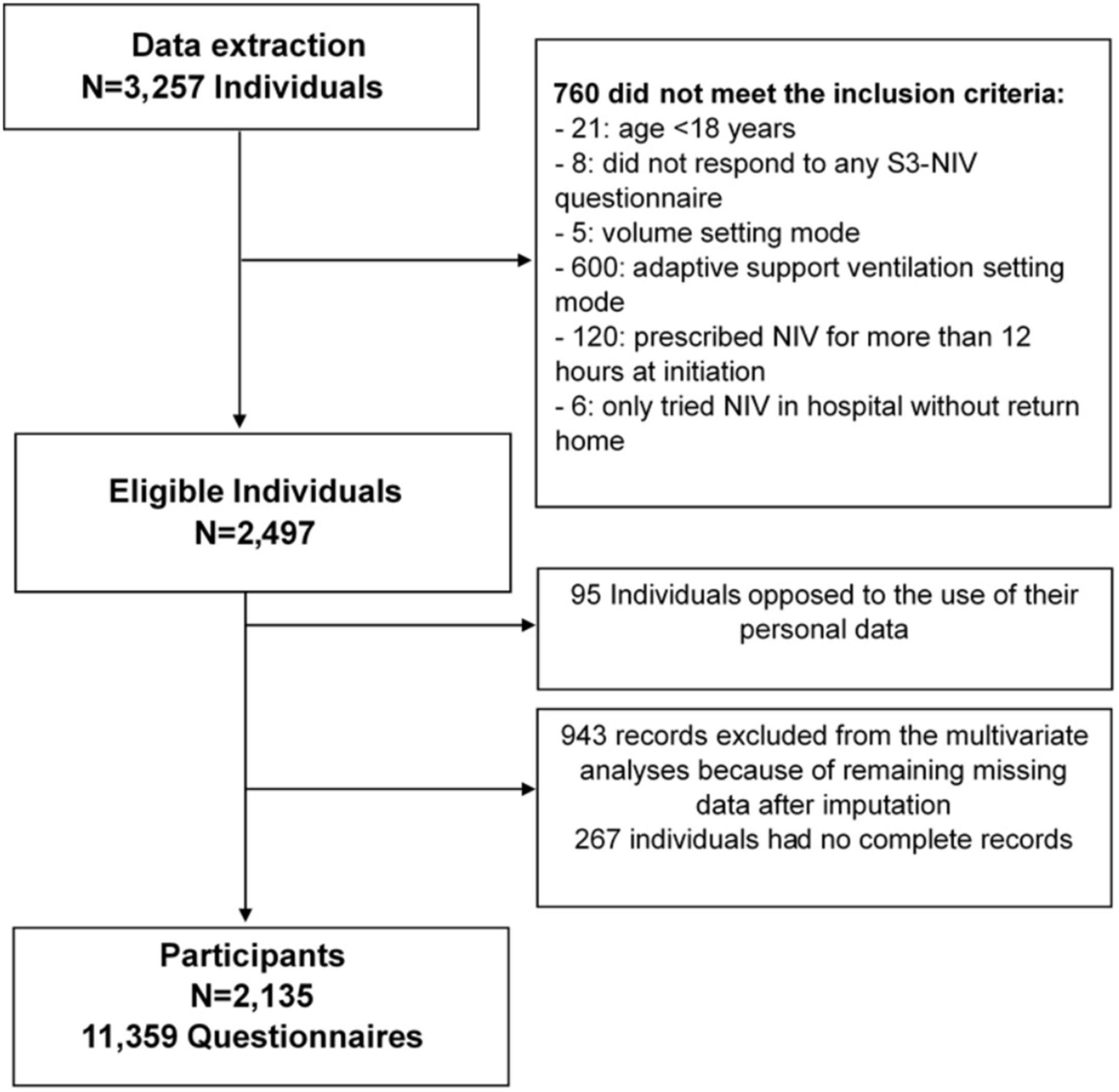
MethodsStudy design and participant selectionThe present work is a retrospective longitudinal analysis of data from a cohort of people with chronic respiratory failure treated at home with long-term, nocturnal, noninvasive ventilation (NIV) who underwent regular assessment between February 2019 and May 2023.
Potential participants were identified through a regional home-care provider database (AGIR à dom). AGIR à dom provides NIV treatment to over 3000 individuals in France, which corresponds to 3 out of every 100 people treated with NIV nationwide.21,22 The inclusion criteria were being aged≥18 years and having completed the S3-NIV questionnaire during a home follow-up visit. Non Inclusion criteria were: (1) NIV prescription for >12h/day (to exclude diurnal use of NIV at the beginning of the study); (2) use of the volumetric mode for the small size of this group (0.16% of the cohort); (3) use of adaptive servo-ventilation (ASV) mode, mainly reserved for patients with central or mixed sleep apnoea syndromes, but not necessarily chronic respiratory failures; and (4) no agreement for the use of their personal data.
The study was carried out in accordance with the European General Protection Regulation (EGPR) and the reference methodology (MR004) of the French information technology and personal data protection authority (Commission Nationale Informatique et Liberté (C.N.I.L)). Before starting the study, a research protocol was drawn up and submitted to the Health-Data-Hub.23 An information letter was mailed to all potential participants to provide them with the opportunity to object to the use of their data. This study is reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) recommendations.24
Data collectionThe following data were retrospectively collected from the homecare provider's database: age, sex, anthropometrics, type of respiratory condition, time elapsed since NIV initiation, NIV settings, supplemental oxygen therapy, adherence (from the device's built-in time counters), and type of interface and S3-NIV scores. For each participant, the first measurement time-point was the date of completion of the first S3-NIV questionnaire. In France, the health authority requires that the home care provider's nurses or technicians visit the individual every three to four months until death or NIV discontinuation. Data for each participant were therefore updated and the S3-NIV questionnaire was completed at each visit.
S3-NIV questionnaireThe S3-NIV questionnaire has 11 items. Each item is scored using a 5-point Likert scale from “always true” to “never true”. The overall S3-NIV score is the average of all items multiplied by 2.5. The S3-NIV evaluates two domains: “respiratory symptoms” and “sleep & side effects”. The score for the first domain is the average score of items 1, 4, 5, 6 and 7 multiplied by 2.5; the score for the latter domain is the average score of items 2, 3, 8, 9, 10 and 11 multiplied by 2.5. The total score and the two sub scores range from 0 to 10 points. Lower scores represent a higher, negative impact of the disease and NIV on the individual.
Statistical analysisQuantitative variables are described by the median and quartiles, and categorical variables by the frequency and percentage.
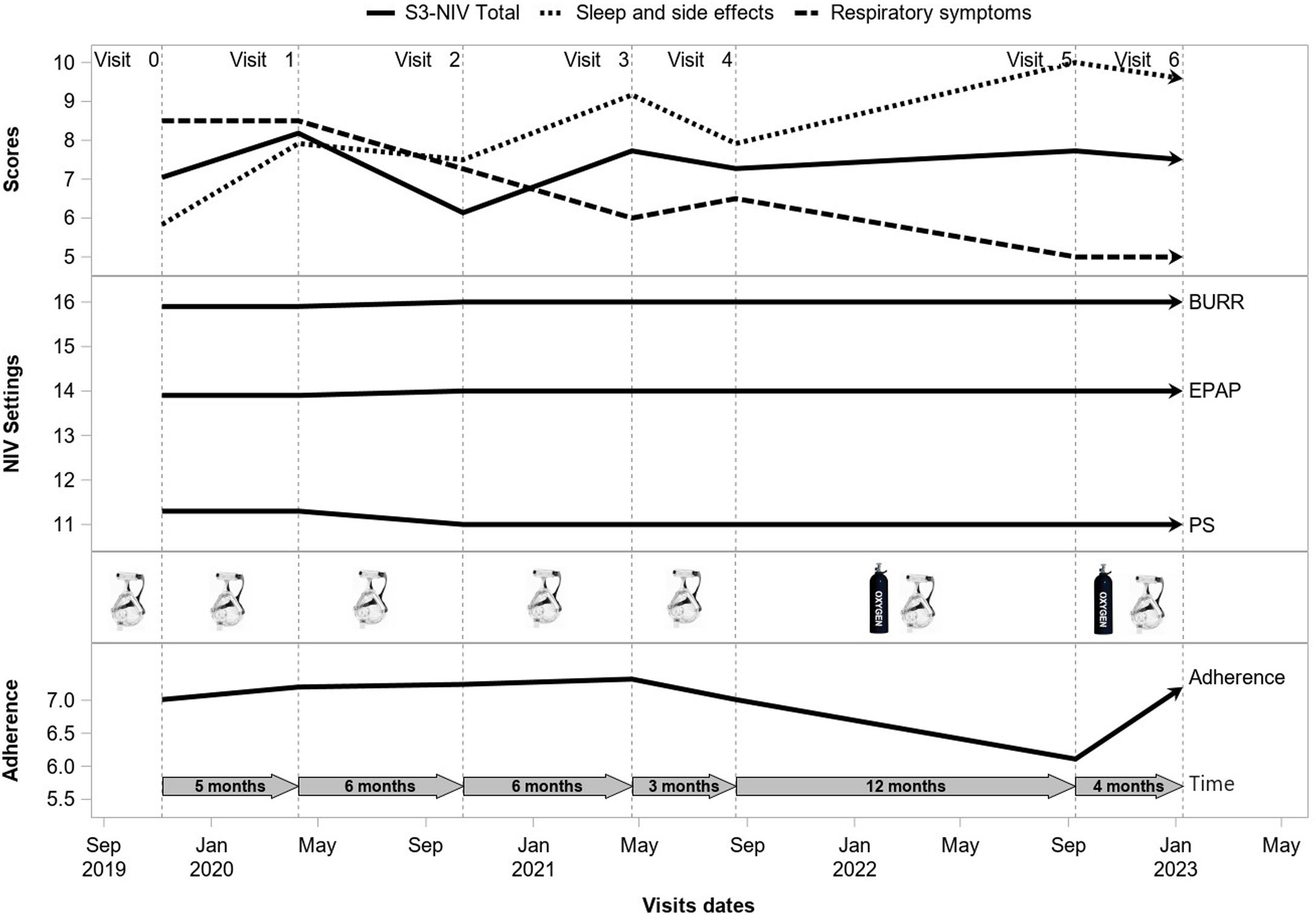
The time-course of the S3-NIV score and its two domains were modeled using multivariate linear mixed effect models. Before creating the models, we assessed the distribution of scores graphically and using the Kolmogorov–Smirnov test. All three models included Individual random effects for both the intercept and the slope. A likelihood ratio test showed that the models with a random-effect of time fitted better than the non-random-effect models (p<0.0001). Time since initiation of treatment, number of provider visits between two visits during which the S3-NIV was completed, sex, age, BMI, type of respiratory condition, type of Interface, adherence (hours/day), oxygen therapy, pressure support and back-up rate were included as fixed effects. Fig. E1 shows an example of the longitudinal follow up of a participant. We chose to include all variables, even those with non-significant effects, to compare their effect on the total score and the sub-scores under the same conditions. No collinearity was detected among the variables that could potentially influence the results. Assessment of residuals confirmed the quality of the three models. Although the sample size has not been calculated, we conducted a post hoc calculation of the statistical power of the study for the equivalence test between the initial and the final S3-NIV scores.it had been estimated to be 0.99 for 2135 participants, considering a minimal clinically significant difference in S3-NIV score from 0.6 to 1.02.20
The change in S3-NIV item scores over time was modeled using a mixed effect multivariable multinomial ordinal regression because of the discrete, ordinal, non-normal distribution of the scores. All item models also included Individual random effects for both the intercept and the slope.
Before statistical analysis, we used intra class correlation (ICC) to check that there were no significant intra-participant changes for the predictor variables: BMI, pressure support, PEP, and back-up rate. Missing data for those variables were imputed from the last records (last observation carried forward). Following the imputation, records with remaining missing values were excluded (Appendix A, Supplementary results).
Statistical computations were performed with SAS for Windows, Version 9.4. p-Values<0.05 were considered significant.
ResultsParticipantsStudy flow chart is depicted in Fig. 1. The median (IQR) follow-up duration was 2 years (10 months–3 years) with a median of 5 (3–7) completed S3-NIV questionnaires per individual. Median time between completions of each S3-NIV questionnaire was 4 months (3–6 months). 595 (28%) participants died during the follow-up; 153 (7%) participants were of lost to follow-up. The reason for their drop-out was not reported.
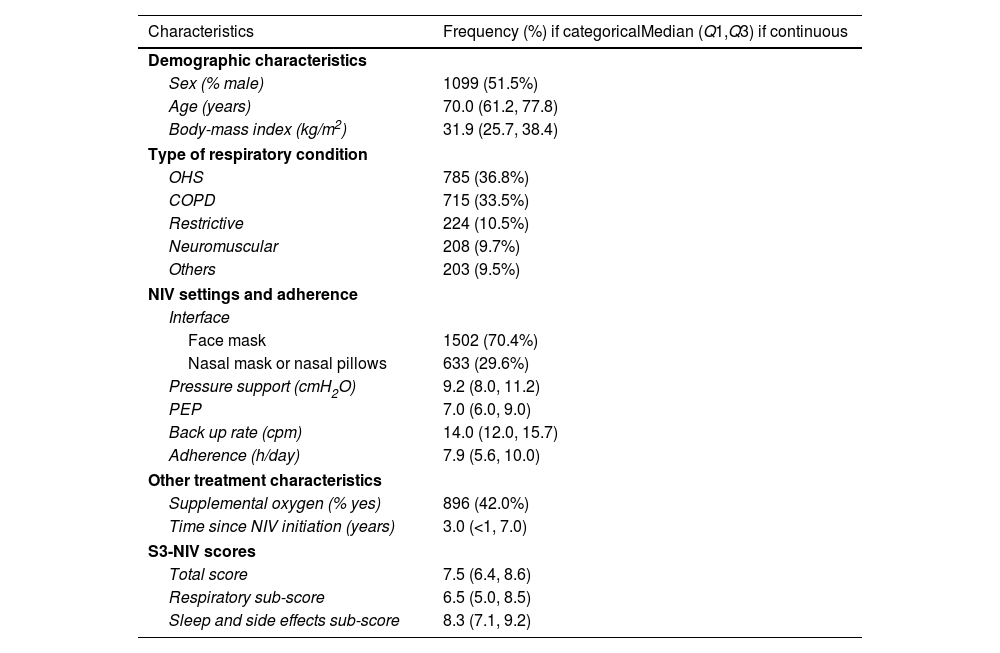
Participants’ baseline characteristics are presented in Table 1. The two most frequent respiratory conditions for which NIV was prescribed were obesity hypoventilation syndrome (OHS) and chronic obstructive pulmonary disease (COPD). At inclusion, these participants had been treated with NIV for a median (IQR) duration of 3 years (7 months–7 years) with a median NIV adherence of 7.9h (5.6–10h/day). Median S3-NIV score was 7.9 (6.4–8.6), with a median respiratory domain score of 6.5 (5.0–8.5) and a median sleep and side effects domain score of 8.3 (7.1–9.2).
Baseline characteristics of participants.
| Characteristics | Frequency (%) if categoricalMedian (Q1,Q3) if continuous |
|---|---|
| Demographic characteristics | |
| Sex (% male) | 1099 (51.5%) |
| Age (years) | 70.0 (61.2, 77.8) |
| Body-mass index (kg/m2) | 31.9 (25.7, 38.4) |
| Type of respiratory condition | |
| OHS | 785 (36.8%) |
| COPD | 715 (33.5%) |
| Restrictive | 224 (10.5%) |
| Neuromuscular | 208 (9.7%) |
| Others | 203 (9.5%) |
| NIV settings and adherence | |
| Interface | |
| Face mask | 1502 (70.4%) |
| Nasal mask or nasal pillows | 633 (29.6%) |
| Pressure support (cmH2O) | 9.2 (8.0, 11.2) |
| PEP | 7.0 (6.0, 9.0) |
| Back up rate (cpm) | 14.0 (12.0, 15.7) |
| Adherence (h/day) | 7.9 (5.6, 10.0) |
| Other treatment characteristics | |
| Supplemental oxygen (% yes) | 896 (42.0%) |
| Time since NIV initiation (years) | 3.0 (<1, 7.0) |
| S3-NIV scores | |
| Total score | 7.5 (6.4, 8.6) |
| Respiratory sub-score | 6.5 (5.0, 8.5) |
| Sleep and side effects sub-score | 8.3 (7.1, 9.2) |
OHS: obesity hypoventilation syndrome; COPD: chronic obstructive pulmonary diseases; CPM: cycle per minute; NIV: noninvasive ventilation; PEP: positive expiratory pressure.
Analysis of change in total S3-NIV, respiratory symptoms domain and sleep and side effects domain scores over time
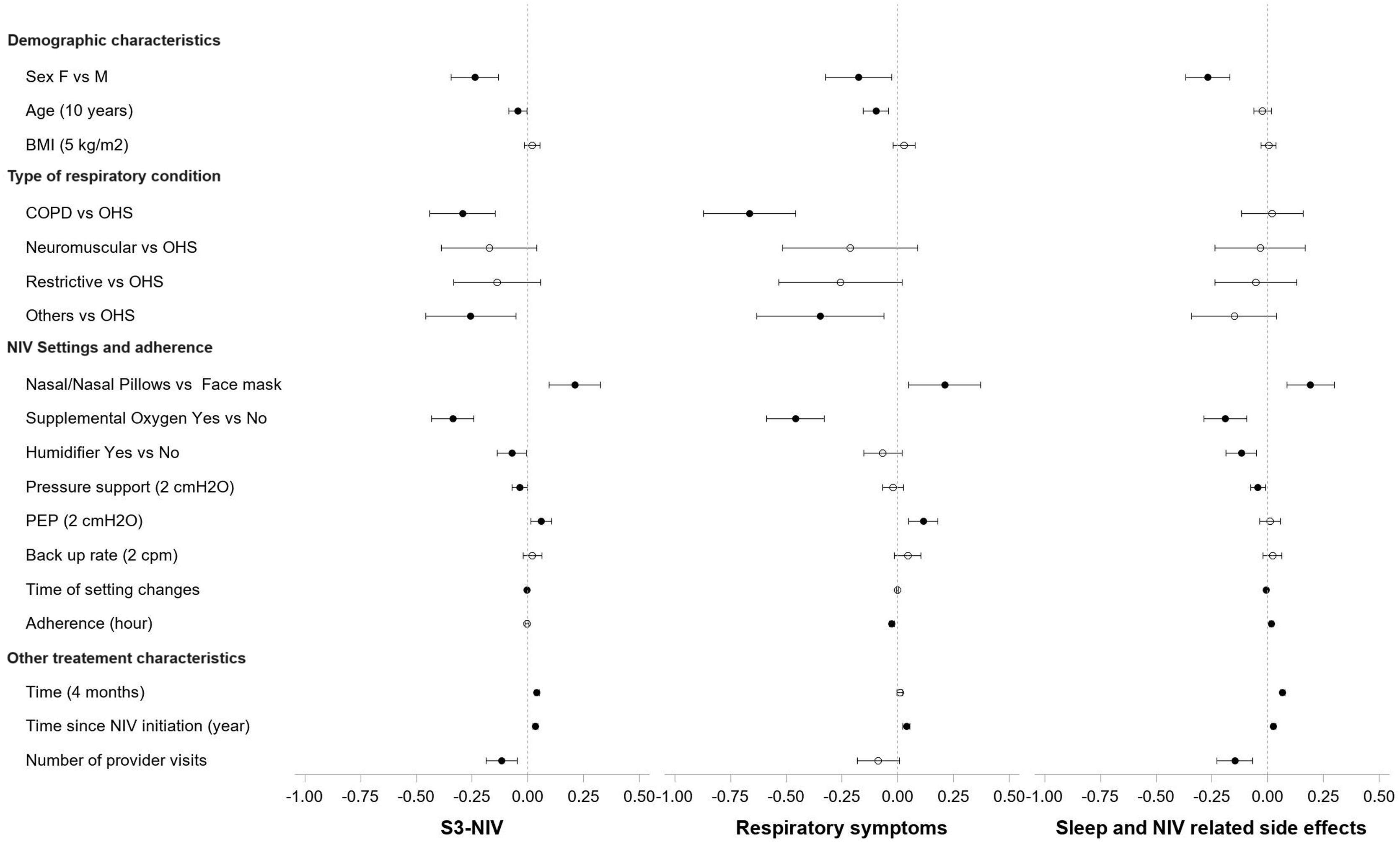
Fig. 2 (and Table S1) shows the variables independently associated with changes in the total score and the two S3-NIV domains. S3-NIV total score was lower for female than male participants over time (β=−0.238, 95% CI: −0.344; −0.132), this was the case for both domain scores (respiratory symptoms: β=−0.176, sleep and side effects: β=−0.268). Older age was associated with lower respiratory symptom scores over time (for each additional 10 years, β=−0.045 95% CI: −0.085; −0.005). BMI was not significantly associated with changes in S3-NIV total score or the domain scores.
Effects Coefficients and Confidence Intervals of predictor variables on the S3-NIV Scores and the two domain scores: respiratory symptoms domain and sleep and side effects domain in multivariate Linear Mixed-Effect Models. COPD: Chronic obstructive pulmonary disease, OHS: Obesity hypoventilation syndrome, PEP: positive expiratory pressure.
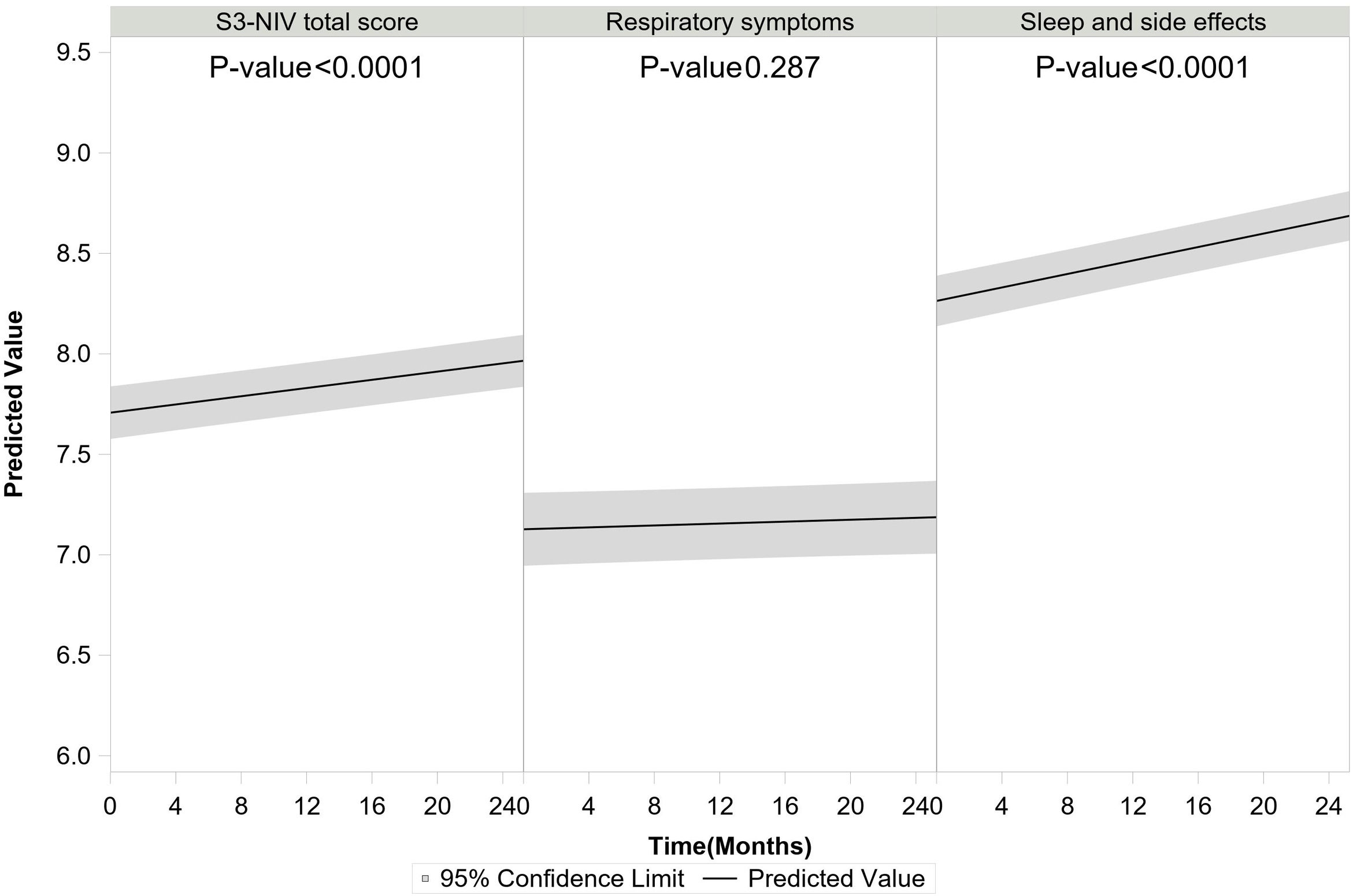
S3-NIV total score improved over time for the whole group (for each additional 4 months, β=0.041 95% CI: 0.032; 0.050). The S3-NIV improvement was mainly associated with positive changes in the sleep and NIV side effects domain (p<.0001) but not the respiratory symptoms domain (p=0.132) (Fig. 3). Time elapsed since initiation of NIV at baseline was also associated with improvement in S3-NIV total score (β=0.035 95% CI: 0.024; 0.046) and the scores of both domains (respiratory symptoms: β=0.039, sleep and side effects: β=0.026) over time. Daily adherence was not significantly associated with change in total S3-NIV score over time (p=0.309), but was associated with a better sleep and NIV side effects score (β=0.017 95% CI: 0.008; 0.025) and a poorer respiratory symptoms score (β=−0.027 95% CI: −0.038; −0.017).
In comparison to participants with OHS, participants with COPD or other respiratory diseases had significantly lower S3-NIV scores over time (COPD vs OHS: β=−0.294 95% CI: −0.441; −0.146, COPD vs OHS: β=−0.256 95% CI: −0.458; −0.054). These differences were associated with a lower respiratory symptoms score, since sleep and NIV related side effects scores did not differ significantly between pathologies.
Participants who used face masks had lower scores over time than those who used nasal/nasal pillow masks (β=−0.210, 95% CI: −0.097; −0.324). Participants who used additional oxygen therapy also had lower scores over time than those who did not (β=−0.337 95% CI: −0.431; −0.242). With regard to NIV settings, higher positive end-expiratory pressure was positively associated with change in S3-NIV total score (for each additional 2cmH2O β=0.06 95% CI: 0.013; 0.01) and with change in the respiratory symptoms domain score (for each additional 2cmH2O β=0.114 95% CI: 0.049; 0.179) over time. Conversely, higher levels of pressure support were associated with lower S3-NIV total scores (for each additional 2cmH2O β=−0.036 95% CI: −0.070; −0.002) and lower sleep and NIV related side effect domain scores (meaning more adverse effects and poorer sleep) over time. Participants provided with humidifier had lower S3-NIV scores over time (β=−0.072 95% CI: −0.137; −0.008), which is mainly due to having more side effects (β=−0.119 95% CI: −0.187; −0.050). The later the changes in NIV settings occurred in the participants’ follow-up, the lower the evolution of the S3-NIV total score over time. This was mainly due to poorer sleep quality and greater adverse effects.
Although the main linear model of the S3-NIV total-score-change over time was adjusted for time since NIV initiation, we performed a sensitivity analysis for participants who had been on NIV for less than a year. The model (Figure Supplement 01) was fairly similar to the main model (including all participants) except for the effect of type of interface. The nasal mask did not appear to be associated with a more positive change of the S3-NIV score than the face mask.
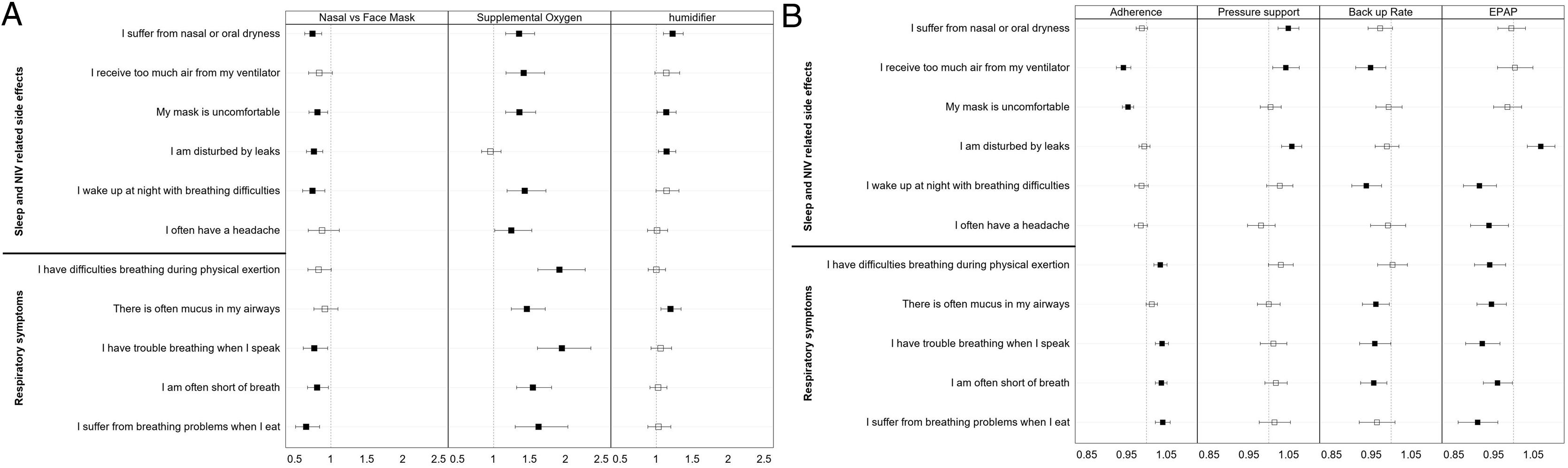
Analysis of changes in S3-NIV individual item scores over timeFig. 4 (and Table S2 supplement) shows the impact of the main NIV settings, type of interface, adherence, the presence of humidifier and additional oxygen therapy on changes in the scores of the 11 items of the S3-NIV questionnaire.
Odds ratio estimates of the variables in the mixed effect multivariable multinomial ordinal models of the 11 items of the S3-NIV questionnaire. (A) Variables Interface, supplemental oxygen and humidifier (B) Variables Adherence, pressure support, back up rate and positive expiratory pressure.
After controlling for confounding variables, perceiving too much air, or experiencing discomfort with the mask was less likely to occur over time in participants with higher levels of adherence. Participants with higher adherence levels were more likely to experience more respiratory symptoms over time, except for mucus in the airways.
In contrast, participants who required higher levels of pressure support were more likely to report NIV related side effects, including nasal and oral dryness, perceiving too much air, being disturbed by leaks, and waking up at night with breathing difficulties over time. However, participants with a higher NIV back up rate were less likely to experience mucus in their airways, have trouble breathing when they speak and shortness of breath. They were also less likely to report receiving too much air or waking up at night with breathing difficulties. Additionally, participants who used higher values of PEP were less likely to experience respiratory symptoms and sleep problems, however they were more likely to report being disturbed by leaks.
Dryness, mask discomfort, disturbing leaks, and waking-up during the night were less likely side-effects for participants who used a nasal mask or nasal pillows than for those who used a face mask. They were also less likely to report shortness of breath or problems breathing when speaking or eating.
Participants who had a humidifier were more likely to report complaints of dryness, mask discomfort, disturbing leaks, and mucus in airways. Participants who had additional oxygen therapy were more likely to report more respiratory symptoms (all those included in S3-NIV), poorer sleep and more NIV side-effects, except for the perception of disturbing leaks.
DiscussionThis study used longitudinal data (up to two years) from a large cohort of individuals undergoing home NIV to identify independent factors associated with changes in S3-NIV scores over time. The type of respiratory condition, sex, age and time elapsed since initiation of NIV was associated with changes in the time-course of the S3-NIV score. Daily NIV adherence level was not associated with a significant change in the S3-NIV total score over time; however, it was associated with poorer respiratory symptoms and improvement in sleep and NIV related side effects over time. Pressure support was associated with a lower total score over time, mainly because side effects increased over time. The use of a face mask (rather than a nasal mask) was associated with a lower S3-NIV total score, with more respiratory symptoms and more side effects over time.
Analysis of the two S3-NIV domain scores provides a more precise interpretation of the time-course of individuals’ symptoms and their perceptions of NIV treatment than analysis of the total score alone. One of the main aims of NIV is to improve the quality of life of people with chronic respiratory failure.4,6,25 Longer NIV daily adherence time is generally considered to be associated with better clinical outcomes.26 However, in the long-term, this association may be complex to interpret, as heavy use of NIV may be associated with a poor prognosis27 or an increase in respiratory symptoms.28 Our results reflect this mechanism: in the presence of severe symptoms, people may increase their use of NIV to relieve the symptoms. However, if they experience considerable side effects, people also reduce their use of NIV. The perception of receiving too much air or mask discomfort are associated with a risk of reduced NIV use, in contrast with the perception of being disturbed by leaks, which is not associated with NIV use. Although the link between leaks perception and non-adherence to continuous positive airway pressure (CPAP) therapy is well-documented in obstructive sleep apnea,29 this association warrants further investigation for NIV.30
The perception of being disturbed by leaks is, however, associated with the level of pressure support, as are the perception of receiving too much air, nasal or oral dryness and waking up with breathing difficulties. Providing sufficient pressure support is obviously essential to reduce hypoventilation, although our results show that pressure support has a considerable impact on NIV-related side effects. Moderation of the level of pressure support can sometimes reduce side effects without reducing the positive impact of the ventilation on hypoventilation.31,32
In contrast with CPAP treatment for sleep apnea syndrome, in which nasal masks are the interface of choice, for NIV, oronasal masks are the most frequently used. Around 60% of people with a thoracic-disease or neuro-muscular disorder and over 80% of people with OHS or COPD use an oronasal mask with NIV.26,33 The reasons for this interface choice in NIV have been sparsely documented. However, our findings suggest that nasal masks are used in the case of mild to moderate respiratory symptoms and are associated with fewer side effects than oronasal masks. However, this was not the case for participants treated for less than a year (sensitivity analysis Fig. S01). A meta-analysis found that people who were fitted with an oronasal mask had more pronounced alveolar hypoventilation at NIV initiation.33 This link between mask type and treatment failure has also been found for CPAP.34 Further research is needed to better rationalize the selection of the interface for NIV.
The proportion of NIV users who have supplemental oxygen therapy varies from one publication to another. Our results show a proportion close to that of another French35 and a Swiss cohort,1 but higher than that of an English cohort.35 In our study, supplemental oxygen therapy was associated with smaller improvements in patient-reported outcomes, with an increase in all symptoms and side effects, except for the perception of leaks. Although the perception of an increase in airway dryness is an expected side effect, the perception of receiving too much air or mask discomfort deserves further investigation. The worsening of all respiratory symptoms over time in those under oxygen supplementation most probably highlights the severity of the disease in this group; however, although oxygen therapy is an essential treatment for severe chronic hypoxemia,36 it is not clearly recommended for moderate hypoxemia with nocturnal or exercise-induced desaturation37–39; nearly 20% of people may receive oxygen therapy despite not fulfilling the criteria for its prescription.40 Unfortunately, in this study we had no information on blood gases or the reason for prescribing O2 to confirm the appropriateness of this treatment, which is a major limitation of this study.
This study has several other limitations. First, the data were collected from a homecare provider database. This database lacks important clinical data that are likely to be related to the change in S3-NIV score including comorbidities, medical treatments, respiratory function, blood gases, or rate of exacerbations; furthermore, The type of respiratory condition that led to the prescription of NIV was mainly administrative (main etiology reported by the physician) with a risk of overlap with other diseases. Second, this study included both people who had recently begun NIV and long-term users. However, we adjusted for time elapsed since NIV initiation. Finally, the modeling of change in the S3-NIV score was conducted with a median follow-up period of only 2 years. A longer follow-up period could potentially enable the identification of other variables that might significantly influence changes in the S3-NIV score over time. It may also possibly reveal different score evolutions according to the type of respiratory condition. However, in the present model, we did not find any significant interaction between type of respiratory condition and adherence.
Conclusion and perspectivesThis study showed that the time-course of the S3-NIV score depends on both the individual's characteristics and the NIV settings. Use of the S3-NIV in clinical practice with separate analysis of the two domains “respiratory symptoms” and “sleep-adverse effects” and of the individual items of the S3-NIV could increase understanding of the difficulties experienced by people on NIV and could serve as a clinical anchor for creating useful alerts with a view to remote monitoring of NIV data.
FundingSB and JLP are supported by the French National Research Agency in the framework of the “Investissements d’avenir” program (ANR-15-IDEX-02) and the “e-health and integrated care and trajectories medicine” from the Grenoble Alpes University Foundation, and “MIAI artificial intelligence” Chairs of excellence (ANR-19-P3IA-0003).
Convention Industrielle de Formation par la Recherche (CIFRE) n° 2022/1389 agreement between l’Association Nationale de la Recherche Technique (ANRT) et Agir à Dom.
Conflict of interestJC Borel, M Lefouili, N Arnol, S Journet, P Chauderon are employees of AGIR à Dom.
Since the initial planning of the work JL Pépin received funding from Air Liquide Foundation, Agiradom, AstraZeneca, Fisher and Paykel, Mutualia, Philips, Resmed, Vitalaire. JL Pépin also received consulting fees from: Agiradom, AstraZeneca, Boehringer, Ingelheim, Jazz pharmaceutical, Night Balance, Philips, Resmed, Sefam.