Bone marrow transplantation (BMT) provides long-term survival. However, the incidence of late-onset non-infectious pulmonary complications is 10%–26%, and can be fatal due to progressive respiratory failure.1 We describe a case of cellular bronchiolitis diagnosed 27 years after allogenic BMT.
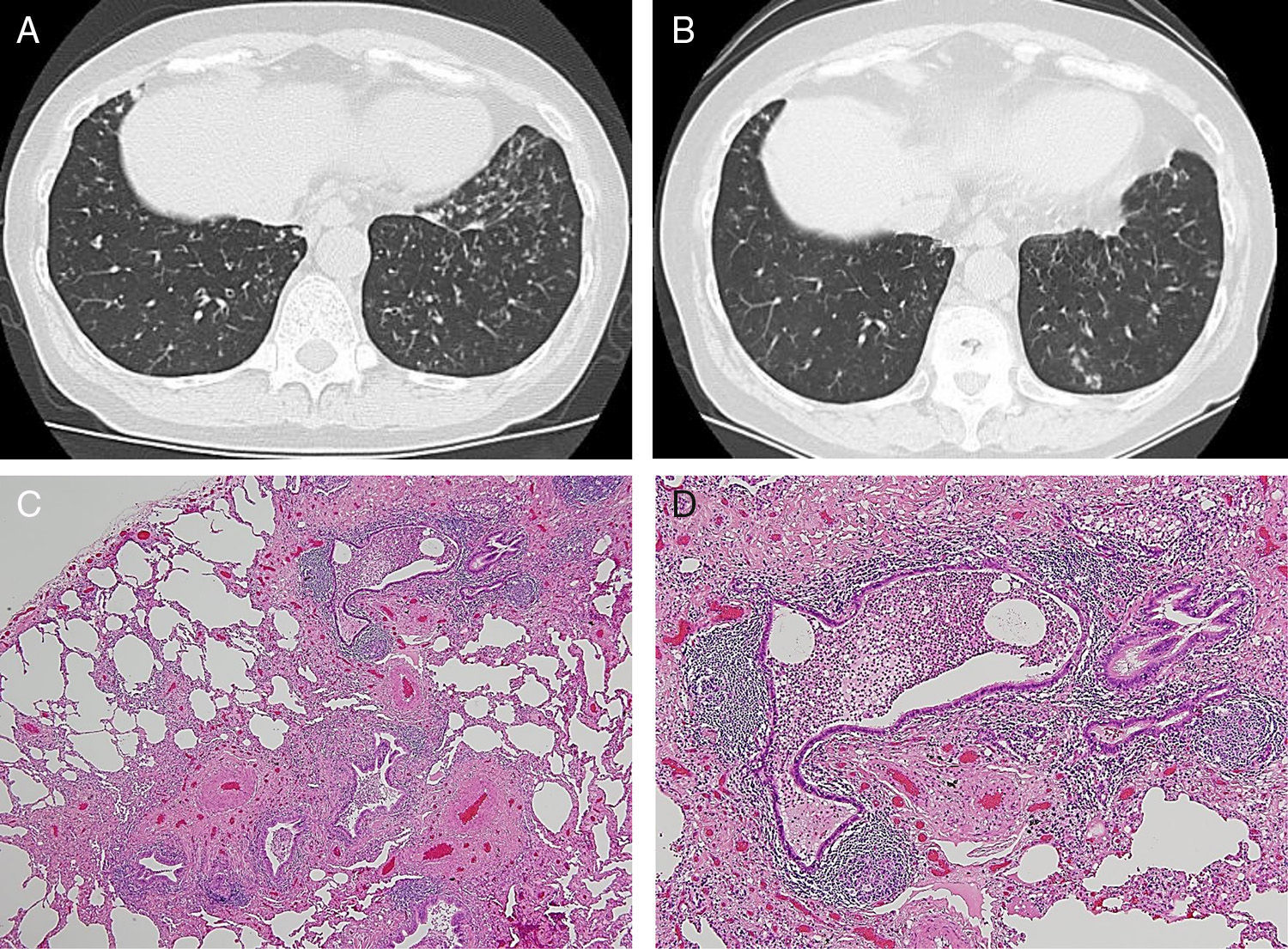
We report the case of a 65-year-old woman, non-smoker, who was diagnosed with acute lymphocytic leukemia at the age of 33 years. After preoperative treatment consisting of busulfan and cyclophosphamide, she received an allogenic BMT from a related donor (her brother), followed by complete remission. At the age of 59 years, she was referred to Aizawa Hospital due to cough and hemoptysis. Thin-section computed tomography (CT) revealed dilated, thick-walled bronchi and tree-in-bud appearance (Fig. 1A). The forced volume capacity (FVC) (%predicted) was 65.3%, and the forced expiratory volume in 1s/FVC was 65.9%. Serologic studies for autoimmune disease (rheumatoid factor, anti-nuclear antibody, SS-A/Ro antibody, and SS-B/La antibody) were all negative. A sputum culture detected Pseudomonas aeruginosa, but no species of mycobacteria. She was treated with 200mg clarithromycin once daily, 10mg montelukast once daily, and one puff of 50μg salmeterol twice daily. Her symptoms of cough and hemoptysis decreased after 1 month. However, after 8 months, CT images had not improved. To determine the nature of her lung disease, open biopsy of the left segments 8 and 9 using video-associated thoracoscopy was performed.
Pathologic specimens (Fig. 1C and D) showed thickening of the wall of membranous bronchioles and the formation of lymphoid follicles with germinal centers due to intraepithelial infiltration of lymphocytes without foamy macrophages. In respiratory bronchioles, reduced intraepithelial infiltration of lymphocytes was observed. However, airway epithelial cell layers were maintained. Therefore, the final pathological diagnosis was cellular bronchiolitis. Thereafter, the patient was treated with three puffs of 160μg budesonide/4.5μg formoterol twice daily, 200mg clarithromycin once daily, and 10mg montelukast once daily. When the thin-section CT images were re-examined 4 years later (at the age of 65), slight improvements were seen (Fig. 1B).
We reported the case of a patient with cellular bronchiolitis, a late-onset non-infectious pulmonary complications diagnosed 27 years after allogenic BMT. In late-onset non-infectious pulmonary complications, bronchiolitis obliterans (BO) occurs in up to 10% of patients undergoing allogenic BMT.1 The clinical features of BO include progressive airflow limitation, and histological findings are epithelial cell necrosis with denudation of mucosa.2 In this case, an obstructive ventilatory defect was detected; however, in pathologic specimens, airway epithelial cell layers were maintained, which is not the typical pathologic findings of BO.
The median development time for late-onset non-infectious pulmonary complications is about 8–12 months after allogenic BMT, although some cases may be diagnosed after 2–3 years.1 This case was diagnosed 27 years after allogenic BMT. This long interval from allogenic BMT raises the possibility that this is a case of an independent disease and not late-onset non-infectious pulmonary complications of BMT. Radiological findings resembled diffuse panbronchiolitis and follicular bronchiolitis. Diffuse panbronchiolitis is characterized histologically by the presence of mononuclear cells and foamy macrophages in the respiratory bronchioles,3 which did not match the histologic findings in our patient. Follicular bronchiolitis is characterized histologically by the presence of hyperplastic lymphoid follicles with reactive germinal centers distributed along the bronchioles,3 which closely resembled this case. Follicular bronchiolitis is usually associated with collagen vascular diseases, particularly rheumatoid arthritis and Sjogren's syndrome.3 Although this patient did not have clinical or serological features of collagen vascular diseases, the appearance of other symptoms should be investigated.
Recently, the efficacy of azithromycin, leukotriene receptor antagonists, inhaled corticosteroids, and bronchodilators on BO has been investigated.4 Our patient was treated with clarithromycin, montelukast, and budesonide/formoterol without systemic corticosteroids because she refused oral corticosteroids; her condition did not vary. In the future, careful attention should be paid to the patient's disease profiles, because the efficacy of clarithromycin, montelukast, and budesonide/formoterol in cellular bronchiolitis as a late-onset non-infectious pulmonary complication has yet to be fully determined.
Advances in BMT technology have led to improvements in long-term survival after transplantation and diminished the risk of developing late complications, as occurred in our patient. Therefore, long-term survivors of BMT should be carefully followed up.
FundingAll authors have no disclosure and financial support regarding this paper.
Conflicts of InterestThe authors state that they have no conflict of interests.
We thank Dr. Masanori Kitaichi for his comments regarding histopathologic findings. We also thank Dr. Kenji Misawa and Dr. Osamu Mishima for performing open biopsy using video-associated thoracoscopy.
Please cite this article as: Hamada S, Yoshioka T, Higuchi K. Bronquiolitis celular: una complicación pulmonar no infecciosa de inicio tardío tras un trasplante alogénico de médula ósea. Arch Bronconeumol. 2017;53:220–221.