At the moment, the best therapeutic hope available for leaving behind the global pandemic produced by the SARS-COV-2 virus is mass vaccination of the world's population.1 Now, More than 150 million individuals infected and more than 3.2 million individuals have died,2 and a significant percentage of survivors of pneumonic episodes will have severe pulmonary sequelae at clinical, functional, radiological and psychological levels.3,4
The first data on infection by this new SARS-COV-2 coronavirus dates back approximately one year, and we have now some evidence of its long-term pulmonary impact, particularly radiological sequelae. Several authors have published their results, based primarily on computed tomography (CT) images of the chest performed weeks or months after the viral pneumonia episode and some of them are already irreversible including: chronic ground-glass opacities, several types of consolidations, reticulation patterns, interlobular septal thickening, crazy paving, honeycombing, adjacent pleural thickening and traction, pulmonary destruction patterns, bronchial wall thickening and pulmonary fibrosis.5
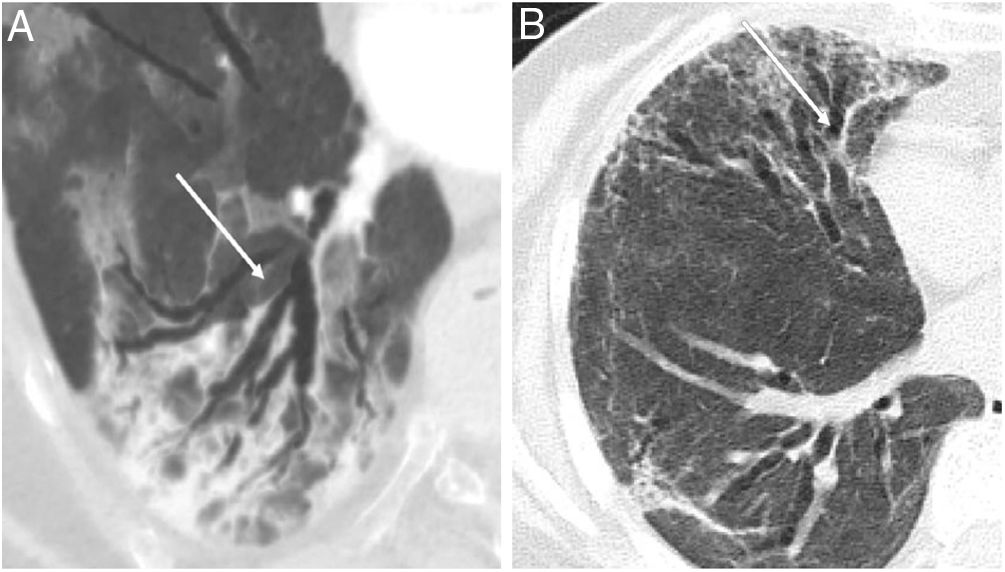
Bronchiectasis, defined as bronchial dilatations with or without associated bronchial wall thickening has been also described as a possible sequelae of pneumonia. Although the appearance of bronchial dilatations has been described in patients who have suffered a COVID-19 pneumonic episode (Fig. 1), even in the acute phase of the disease,6,7 in most of the cases have been observed months after the COVID-19 infection. One very recent study that analyzed the radiological changes (according to CT scan) at six months of 114 survivors of severe pneumonia due to COVID-19 observed that approximately one third of the patients developed fibrotic-like changes in the post-pneumonic lung, and that bronchiectasis was present in 24% of the patients at follow-up, compared with only 7% in the acute stage of the disease. These findings suggest that bronchiectasis (at least from a radiological point of view) can appear in up to a quarter of patients who suffered from severe COVID-19 pneumonia.5
(A) Radiological bronchiectasis (white arrows) in the acute infectious disease (pneumonia) and, (B) radiological bronchiectasis in the fibrotic phase of the infection (free access from Refs. 5 and 6).
Although this is a relevant finding since it probably implies a permanent alteration to the lung parenchyma, different circumstances should be highlighted. On the one hand, the percentage of patients who already had bronchiectasis prior to COVID-19 pneumonia, as well as the role of the viral infection in determining them are not known. On the other hand, the etiology of these dilated airways is also relatively unknown, in many cases may be due to traction in the context of pulmonary fibrosis. This hypothesis is supported by the results obtained by Han et al.,5 who observed that the majority of patients who develop bronchiectasis also present with pulmonary fibrotic changes presumably secondary to the COVID-19 pneumonia. In this respect, the nature of the inflammatory component of this bronchiectasis, its potential to lead to recurrent bronchial infection and inflammation by potentially pathogenic microorganisms and, therefore, its potential to worsen prognosis, are unknown. Likewise, it is unknown whether this bronchiectasis is merely a radiological finding or whether it can be associated to respiratory symptoms requiring and progressive change over time. Finally, the specific prognostic factors for the development of bronchiectasis are also unknown, although in this same study the authors observed that the following were prognostic factors for subsequent fibrotic sequelae: age greater than 50 years; heart rate at admission higher than 100bpm; more than 17 days of hospitalization; a need for non-invasive mechanical ventilation; presence of an acute respiratory distress syndrome, and a greater initial involvement on CT scan.5 We can speculate that some of these risk factors could be also considered as relevant for the development of bronchiectasis in these patients but this question requires additional investigation. However, the potential of these findings should not be underestimated given the known association between a history of viral infections, including measles, respiratory syncytial virus, influenza virus and human immunodeficiency virus and permanent bronchiectasis. In this sense, Chen et al. reported that 25% of survivor patients developed bronchiectasis 12 months after the epidemic influenza A (H7N9) virus infection.8 Surprisingly, there is no information about the development of bronchiectasis up to 15 years after the infection by the CoV-1, although the fibrotic changes on CT scan have been reported.9 Thus, although the causal relationship between the COVID-19 pneumonia and the subsequent production of clinically relevant bronchiectasis has not yet proven, it provided an important breeding ground for research, which should undoubtedly be based on the large national and international longitudinal registries of COVID-19 and bronchiectasis now in existence throughout the world. The findings and questions presented so far in the literature support the need for longitudinal monitoring, including by serial CT scan, of those patients who have suffered severe COVID-19 pneumonia.