Most areas of respiratory medicine continue to use an Oslerian approach, based on signs and symptoms, in which the disease is the center of all activity. However, this paradigm is changing. Now that lung diseases have been recognized as heterogeneous and complex, we are moving toward more personalized, precise, patient-oriented medicine. The aim of this review was to define the current state of the knowledge on bronchiectasis, or, more accurately, the bronchiectasis syndrome, as a multidimensional, systemic, heterogeneous, complex disease. We explore the advances that have already been made, and above all the many steps that are still to be taken. We also propose some tools which might facilitate the application of these concepts in clinical practice, and help us continue our journey toward a more holistic view of this disease.
En la mayoría de los ámbitos de la neumología se sigue utilizando un principio osleriano (basado en los síntomas y signos) en los que la enfermedad es el centro de toda actividad, pero este paradigma está cambiando. Actualmente, gracias al reconocimiento de la heterogeneidad y complejidad de las enfermedades pulmonares, la tendencia es a realizar una medicina más personalizada, de precisión, o centrada en el paciente. En la presente revisión se intentará establecer la situación actual sobre el conocimiento de las bronquiectasias, o mejor, del síndrome bronquiectásico, como una enfermedad multidimensional, sistémica, heterogénea y compleja, los pasos que ya se han dado en este sentido, y sobre todo, en los muchos que quedan por dar. Asimismo, se propondrán algunas herramientas que podrían facilitar la traslación de estos conceptos a la práctica clínica, y con ellos seguir avanzando hacia una imagen más holística de esta enfermedad.
A large part of the diagnosis and treatment of diseases in practice today still relies on the old Oslerian tradition, based on pathological signs and symptoms (syndrome) and a small number of complementary tests and therapeutic regimens.1 Nevertheless, advances in the understanding of molecular and pathophysiological pathways make it clear that diseases are highly complex entities, and our current approach to diagnosis and treatment is extremely reductionist. Furthermore, diseases do not affect individuals in a unique way, but instead present in a variety of forms, depending on the particular personal or environmental circumstances of each subject, and are ultimately the result of the interaction between genetics and the environment.2 Diseases can also be associated to some extent with other diseases occurring in the same individual, and they can change over time or as a result of the treatment administered. We cannot yet control all these characteristics, but such an approach will most likely to bring us closer to real precision medicine,3 the ultimate aim of which is to tailor treatment to the characteristics of each individual.
Diseases of the airways, and in particular bronchiectasis, are no exception to this rule.2 In this review, we seek to address current knowledge of both the complexity and the heterogeneity of bronchiectasis as the first step toward developing tools that will smooth the way toward precision medicine in this disease in the future.
Bronchiectasis is a Heterogeneous DiseaseBronchiectasis is a heterogeneous disease in many ways, if we take heterogeneity to mean that not all variables that define the disease appear in all individuals at the same time.2,4 A key aspect to understanding the heterogeneity of bronchiectasis stems from its very origin, given that what we refer to today as bronchiectasis is just the final stage of the pulmonary component of more than a hundred diseases, both local and systemic.5,6 Moreover, each patient may present with different clinical, prognostic, and radiological characteristics, and possibly even different therapeutic responses, so “bronchiectasis” is in fact an umbrella term for a wide group of diseases.
How Can We Deal With Heterogeneity in Bronchiectasis?Current Concept of BronchiectasisThe first factor to take into account is that bronchiectasis (which by definition means “bronchial dilation”) is not exclusively a lung disease, so it is probably more correct to speak of a “bronchiectasis syndrome”.2 It is accepted, then, that bronchial dilation observed on high-resolution computed tomography (HRCT) must be accompanied by consistent clinical symptoms (usually chronic productive cough).7 Thus, the definition of bronchiectasis should ideally exclude purely radiological traction-induced bronchial dilation, or other forms that appear in almost 20% of healthy individuals of advanced age.8,9 Finally, the best radiological criterion for the diagnosis of bronchiectasis has yet to be defined. The long-standing Naidich criteria9 (based on a bronchoarterial ratio >1) are still the most widely used and are currently recommended by the SEPAR guidelines10 However, some authors have observed that in patients with other lung diseases (such as chronic obstructive pulmonary disease [COPD]) the diameter of the adjacent vessel is sometimes increased due to vascular hypertension or (more commonly) reduced due to hypoxic vasoconstriction, which can lead to underdiagnosis or overdiagnosis of bronchiectasis, respectively, when these criteria are used.11,12
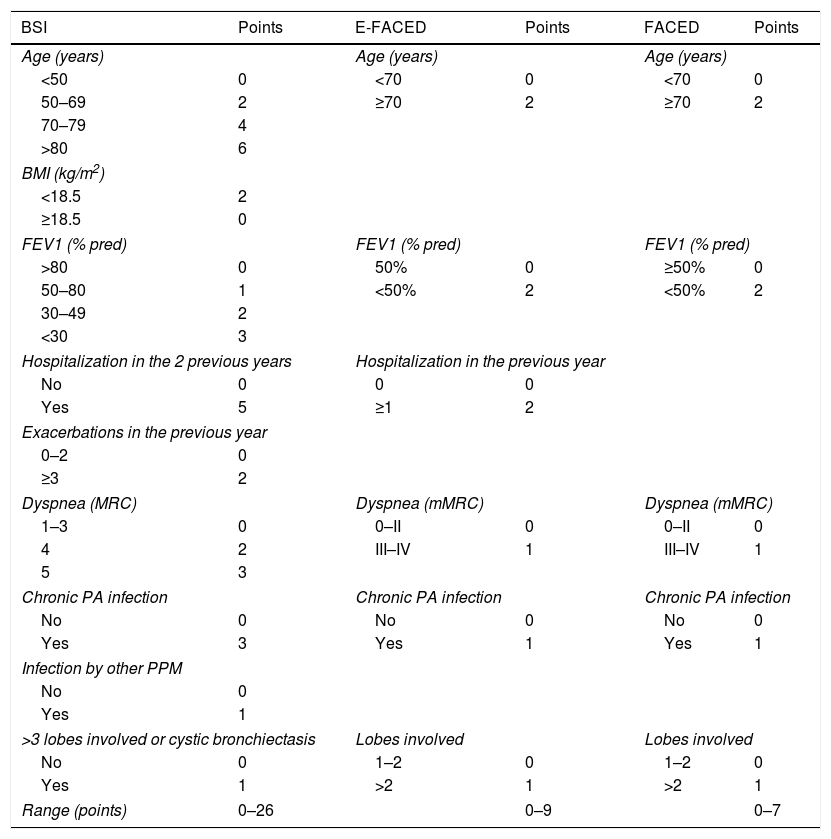
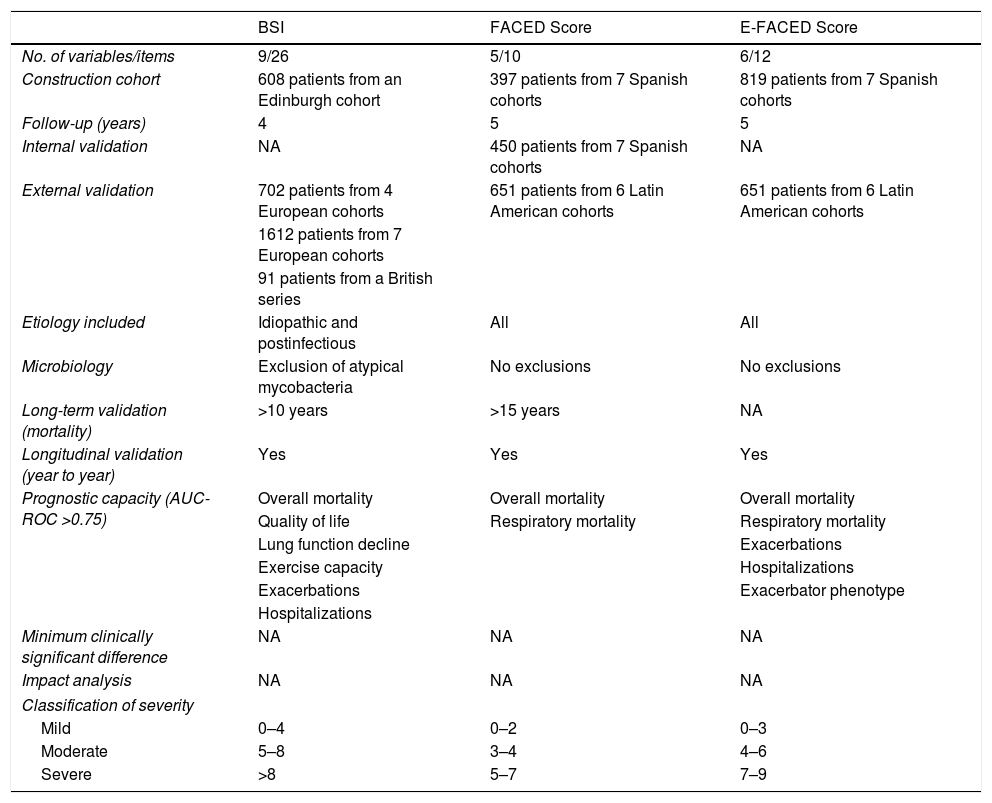
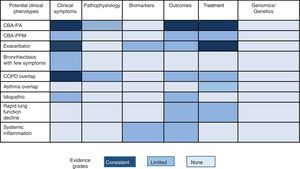
Multidimensional ScoresMultidimensional scores have recently been developed to assess the severity of bronchiectasis, carefully taking into account a series of important pathological dimensions. In the case of bronchiectasis, these include symptoms (dyspnea and exacerbations), lung function, microbiology, radiological aspects, age, and other anthropometric variables. Table 1 shows the composition and description of the 3 best validated scales currently described in the literature: E-FACED13 (an acronym for Exacerbations, FEV1, Age, chronic Pseudomonas aeruginosa bronchial infection [Colonization], radiological Extension, and Dyspnea), FACED,14 and the Bronchiectasis Severity Index (BSI).15 Thanks to its simplicity and its excellent internal and external validation, E-FACED is recommended by the current SEPAR guidelines for the management of bronchiectasis.10 The characteristics of each of the scales mean that their usefulness may depend on what exactly we want to assess and the setting in which it is intended to be used. Table 2 shows the comparative characteristics of the 3 scores.
BSI, FACED and E-FACED. Quantification Variables and Description.
| BSI | Points | E-FACED | Points | FACED | Points |
|---|---|---|---|---|---|
| Age (years) | Age (years) | Age (years) | |||
| <50 | 0 | <70 | 0 | <70 | 0 |
| 50–69 | 2 | ≥70 | 2 | ≥70 | 2 |
| 70–79 | 4 | ||||
| >80 | 6 | ||||
| BMI (kg/m2) | |||||
| <18.5 | 2 | ||||
| ≥18.5 | 0 | ||||
| FEV1 (% pred) | FEV1 (% pred) | FEV1 (% pred) | |||
| >80 | 0 | 50% | 0 | ≥50% | 0 |
| 50–80 | 1 | <50% | 2 | <50% | 2 |
| 30–49 | 2 | ||||
| <30 | 3 | ||||
| Hospitalization in the 2 previous years | Hospitalization in the previous year | ||||
| No | 0 | 0 | 0 | ||
| Yes | 5 | ≥1 | 2 | ||
| Exacerbations in the previous year | |||||
| 0–2 | 0 | ||||
| ≥3 | 2 | ||||
| Dyspnea (MRC) | Dyspnea (mMRC) | Dyspnea (mMRC) | |||
| 1–3 | 0 | 0–II | 0 | 0–II | 0 |
| 4 | 2 | III–IV | 1 | III–IV | 1 |
| 5 | 3 | ||||
| Chronic PA infection | Chronic PA infection | Chronic PA infection | |||
| No | 0 | No | 0 | No | 0 |
| Yes | 3 | Yes | 1 | Yes | 1 |
| Infection by other PPM | |||||
| No | 0 | ||||
| Yes | 1 | ||||
| >3 lobes involved or cystic bronchiectasis | Lobes involved | Lobes involved | |||
| No | 0 | 1–2 | 0 | 1–2 | 0 |
| Yes | 1 | >2 | 1 | >2 | 1 |
| Range (points) | 0–26 | 0–9 | 0–7 | ||
BQ: Bronchiectasis; BMI: body mass index; BSI: Bronchiectasis Severity Index; E-faced: acronym for Exacerbations, FEV1, Age, chronic Pseudomonas aeruginosa bronchial infection (Colonization), radiological Extension, and Dyspnea; mMRC: Modified Medical Research Council; PA: Pseudomonas aeruginosa; PPM: potentially pathogenic microorganisms.
Comparative Table of the Main Characteristics of Existing Multidimensional Scores.
| BSI | FACED Score | E-FACED Score | |
|---|---|---|---|
| No. of variables/items | 9/26 | 5/10 | 6/12 |
| Construction cohort | 608 patients from an Edinburgh cohort | 397 patients from 7 Spanish cohorts | 819 patients from 7 Spanish cohorts |
| Follow-up (years) | 4 | 5 | 5 |
| Internal validation | NA | 450 patients from 7 Spanish cohorts | NA |
| External validation | 702 patients from 4 European cohorts | 651 patients from 6 Latin American cohorts | 651 patients from 6 Latin American cohorts |
| 1612 patients from 7 European cohorts | |||
| 91 patients from a British series | |||
| Etiology included | Idiopathic and postinfectious | All | All |
| Microbiology | Exclusion of atypical mycobacteria | No exclusions | No exclusions |
| Long-term validation (mortality) | >10 years | >15 years | NA |
| Longitudinal validation (year to year) | Yes | Yes | Yes |
| Prognostic capacity (AUC-ROC >0.75) | Overall mortality | Overall mortality | Overall mortality |
| Quality of life | Respiratory mortality | Respiratory mortality | |
| Lung function decline | Exacerbations | ||
| Exercise capacity | Hospitalizations | ||
| Exacerbations | Exacerbator phenotype | ||
| Hospitalizations | |||
| Minimum clinically significant difference | NA | NA | NA |
| Impact analysis | NA | NA | NA |
| Classification of severity | |||
| Mild | 0–4 | 0–2 | 0–3 |
| Moderate | 5–8 | 3–4 | 4–6 |
| Severe | >8 | 5–7 | 7–9 |
BSI: Bronchiectasis Severity Index; E-FACED: acronym for Exacerbations, FEV1, Age, chronic Pseudomonas aeruginosa bronchial infection (Colonization), radiological Extension, and Dyspnea; NA: not available.
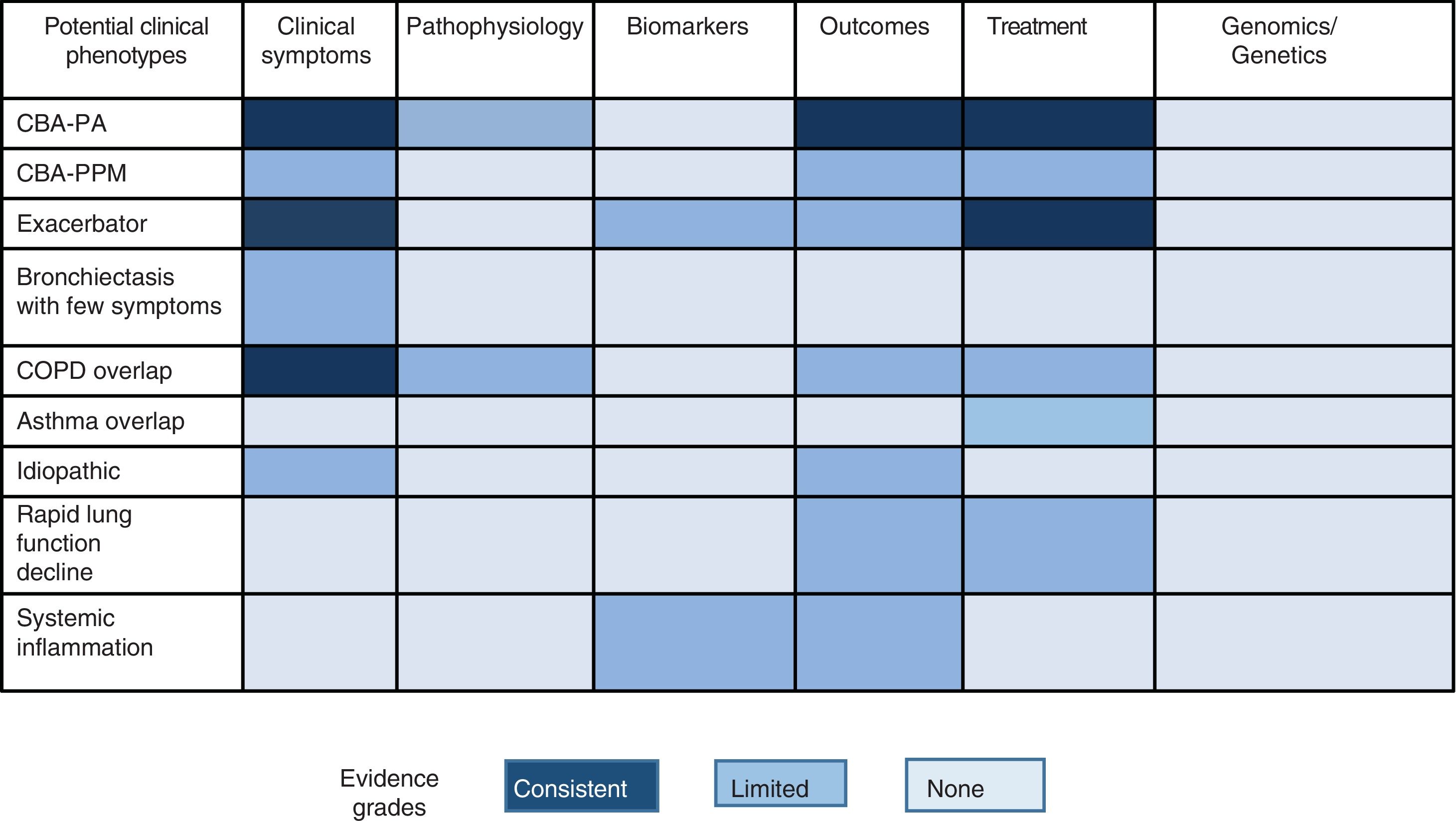
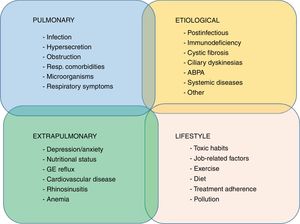
Some studies have tried to pool patients with more homogeneous clinical or prognostic features, known as clinical phenotypes, in an attempt to offer a more personalized treatment and follow-up (stratified medicine). Given the enormous heterogeneity of bronchiectasis, some authors have achieved this goal by using statistical techniques such as cluster analysis. Aliberti et al.16 analyzed 1145 patients from the European bronchiectasis cohort and observed 4 clusters or groups of patients with similar characteristics that could be differentiated from the other groups: (1) patients with chronic bronchial Pseudomonas aeruginosa (PA) infection; (2) patients with chronic bronchial infection due to other potentially pathogenic microorganisms (PPM); (3) patients with abundant expectoration, and (4) patients with few symptoms. For their part, Martinez-Garcia et al.,17 using a similar methodological technique in 468 patients from different Spanish cohorts, also noted 4 distinct clusters: (1) young patients with clinically mild bronchiectasis; (2) elderly patients with few symptoms; (3) elderly patients with frequent attacks; and (4) elderly patients with few exacerbations. As in the previous study, these groups could be differentiated in terms of disease severity, etiology, presence of chronic bronchial infection, and mortality.
It is clear, however, that the “phenotyping” of patients with bronchiectasis is only an intermediate step toward obtaining a complete picture of the heterogeneity of the disease, since there is still much to understand, such as the existence of other unidentified phenotypes, the presence of several of these phenotypes in the same patient, phenotype stability over time, and, in particular, response to a certain pathophysiological mechanism (endotype) and specific treatment.18
Other studies have focused on the analysis of supposed specific clinical phenotypes in which a different clinical, prognostic or therapeutic behavior has been observed, according to the definition proposed by Han et al.19 Of these, perhaps 3 have generated the most clinical evidence to date: patients with chronic bronchial PA infection, patients with COPD overlap syndrome, and the exacerbator phenotype.
Chronic P. aeruginosa Bronchial InfectionThis is clearly the best characterized phenotype. Various studies show that the presence of PA in the airways of patients, especially those with chronic symptomatic bronchial infection, is associated with greater clinical severity, poorer quality of life, more numerous and more severe exacerbations, worse prognosis, and specific treatment.20–25 Some key aspects remain to be clarified, such as the natural history of patients with asymptomatic chronic PA bronchial infection, or the endotype that underlies this phenotype, given that it remains unclear whether PA infection is the cause or the consequence of this worse prognosis.26 Finally, these patients are an ideal target for the investigation of new specific drugs.27
COPD-bronchiectasis Overlap SyndromeThis is probably the best characterized phenotype after chronic PA bronchial infection. Its epidemiological importance is obvious given that it encompasses 2 widely prevalent diseases.28 More than 20 studies in the literature show a higher-than-expected prevalence of bronchiectasis in patients with COPD (especially in severe cases).29 These patients have more frequent and more severe exacerbations and their clinical status is more severe.30 More controversial is the role of bronchiectasis in the prognosis of patients with COPD and the existence of a causal link between both entities, although the data published to date suggest that it is biologically plausible that patients with COPD and chronic bronchial infection by PPM could generate bronchiectasis, and that these conditions will worsen patient prognosis.30 Finally, these patients require combined treatment of both conditions following the recommendations of the corresponding guidelines.23–25,31,32 Many other aspects remain to be clarified, especially those that raise the possibility of preventing the development of bronchiectasis by promptly treating chronic bronchial infection in COPD patients.
Frequent ExacerbatorExacerbations, especially when severe, have been shown to have an impact on the prognosis of patients with bronchiectasis. There is a particular group of patients who continue to present frequent exacerbations every year despite treatment, although this is a little studied phenotype. These individuals present some singular clinical features. The best cut-off point in the number of exacerbations to consider the presence of this phenotype is unknown. Two studies stand out in this regard. Chalmers et al.33 observed a worse prognosis in patients with a greater number of exacerbations and found that this phenotype remained stable over time, that is to say, the parameter that best predicted a future exacerbation was the number and severity of exacerbations experienced in the past. On the other hand, a recent prospective study by Martinez-Garcia et al.34 showed that, among all the possible combinations, the parameter consisting of at least 2 exacerbations or 1 hospitalization for exacerbations per year was associated with a worse prognosis, and that the E-FACED score was able to predict their appearance.34 According to current guidelines, more specific treatments are available for these patients, such as macrolides, hypertonic formulations, or different combinations of antibiotics.
Other possible phenotypes, not yet explored in depth, that may be candidates for future studies include dry bronchiectasis (bronchiectasis with few symptoms), bronchiectasis with systemic inflammation, idiopathic forms, chronic bronchial infection due to micro-organisms other than PA, or rapidly declining lung function.35 It is important to note that the existence of different phenotypes should not be confused with severity, activity or impact of the disease on the patient (these 3 factors can range widely in each clinical phenotype, and will be discussed in the next section).
Fig. 1 shows the different proposals, graded by color, for possible clinical phenotypes (not confirmed) and the greater or lesser scientific evidence available (more evidence is indicated by a more intense color).
There are also other variables that, while not strictly constituting a clinical phenotype, can alter the phenotypic expression of the patient: these could be called modifier variables: age, sex, comorbidities36,37 (obesity, anxiety/depression, etc.), etiology, and socioeconomic status.
Bronchiectasis is a Complex DiseaseThe complexity of a disease is determined by the non-linear and dynamic relationships that exist between its different components.38 Non-linearity is understood as the lack of proportionality in the changes that occur among these variables. One example might be a patient with bronchiectasis who frequently presents a dissociation between clinical-functional and radiological findings.39 Therefore, the complexity of a disease is much more difficult to control than heterogeneous presentation, because its existence ultimately depends on genomic interactions (about which very little is known in bronchiectasis) and the multiple environmental factors to which the patient is exposed. However, it is enormously important to gain as much knowledge as possible about this complexity because, at the end of the day, it forms the basis of true precision medicine in bronchiectasis (as in any other disease).
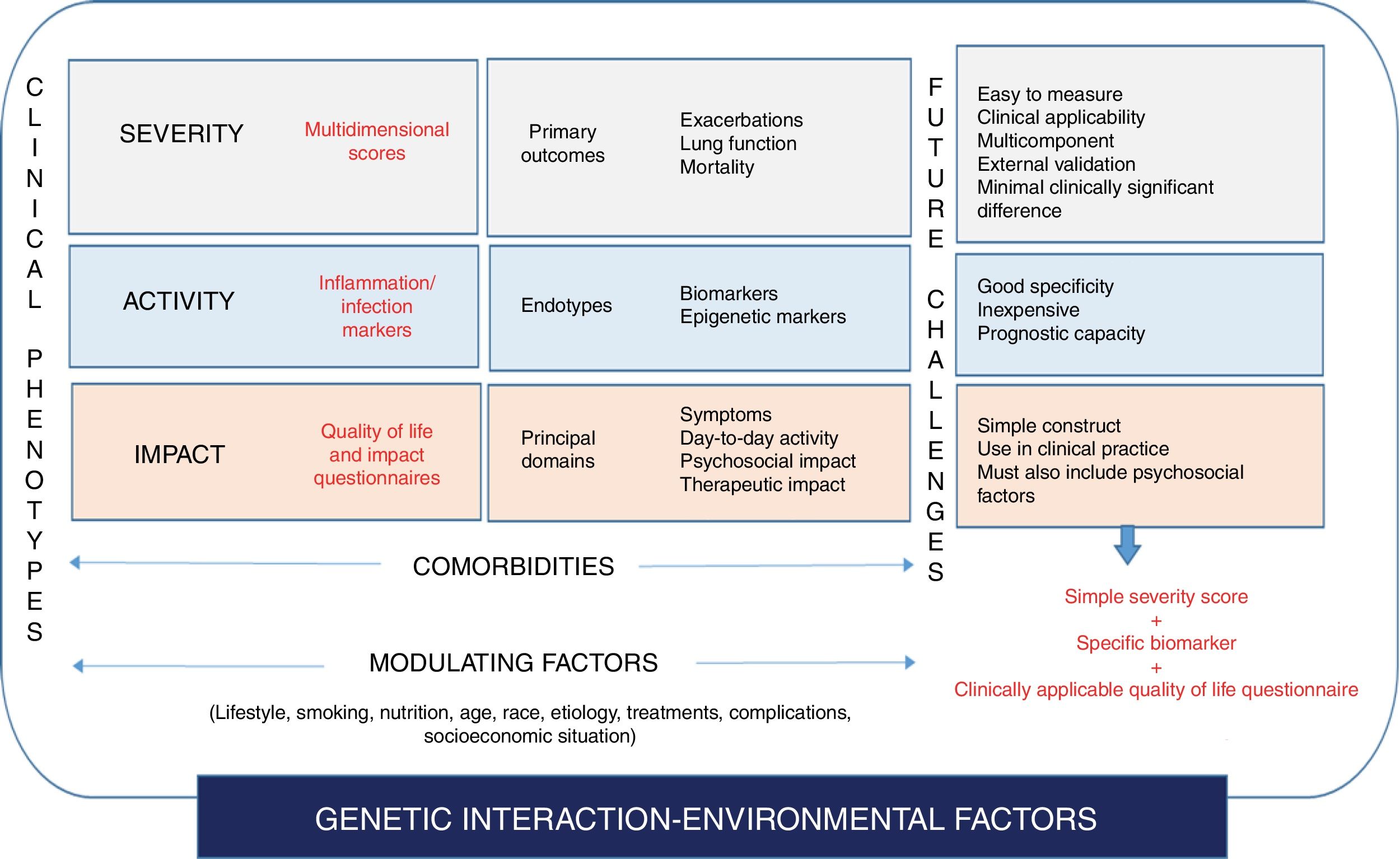
An initial approach to the complexity of bronchiectasis would be to identify the key dimensions of the disease, or the factors that provide information independent from what we know as the bronchiectasis syndrome: its severity, its (biological) activity, and its impact on the patient.
SeverityThis dimension would consist of identifying and measuring variables associated with the damage that the disease causes in the affected target organs. Important variables in the case of bronchiectasis would be: lung function changes (FEV1 is probably most widely accepted marker of airflow obstruction),13–15 structural lung damage (extension on HRCT),9 the etiology of the disease,40 or comorbidities that can potentially modify the disease course and which can be quantified using various tools, such as the Charlson index,41 or the Bronchiectasis Etiology and Comorbidities Index (BACI).42
ActivityThis parameter refers to the level of biological activation of the disease at a certain point in time, and is independent from severity.43,44 “Activity”, therefore can be measured using a biomarker (biological or otherwise), and will be closely related with the endotype substrate, which in the case of bronchiectasis would focus on the inflammation-infection binomial.45 Some markers of inflammation-infection are relatively easy to obtain and are used in daily practice to measure disease activity, such as the Murray sputum purulence scale)46 and sputum volume (quantitative assessment). Another factor that can also mark disease activity is systemic inflammation itself, a feature that has been associated with a greater degree of local inflammation and severity.47 However, no specific, valid biomarker has been identified yet, although C-reactive protein48 or neutrophil elastase49 could prove useful in the future. Finally, the number and severity of exacerbations may also be considered a parameter of uncontrolled disease activity despite treatment.33,34 For this reason it is very important to record this data, and exacerbator patients could constitute a special phenotype with prognostic peculiarities of their own.
ImpactThe impact of the disease on the individual is a crucial factor, and the quantification of impact is also independent of severity and the biological activity of the disease. Questionnaires evaluating quality of life, cough, and symptoms, such as dyspnea, or psychiatric disorders, such as anxiety and depression, are fundamental in this regard. Different tools are available for all these variables, some of which are specific for bronchiectasis, such as the quality of life questionnaires Bronchiectasis Health Questionnaire,50 QoL-B,51,52 Leicester Cough Questionnaire,53 and others are generic assessments of anxiety/depression, such as the Hospital Anxiety-Depression Scale (HADS),54 or dyspnea (using the Medical Research Council scale [MRC]).55 One study showed how the degree of anxiety/depression in bronchiectasis, which has been very little studied to date,56 does not depend on the severity of the disease measured by the conventional multidimensional scores.
Finally, all these variables can also be modulated by modifying factors such as age, sex, geographical origin, race and other environmental factors, such as drug interactions or patient lifestyle, all of which add to the complexity to the situation.
Bronchiectasis. A Holistic View of the DiseaseTo the extent permitted by current knowledge and technology, a double proposal could be made that brings both heterogeneity and complexity (including therapeutic considerations) of bronchiectasis closer to a more holistic approach to the disease, one that is more inclusive in terms of treatment and more representative of true precision medicine.
It would be interesting to focus efforts on future easy-to-use tools that integrate severity, activity, and the impact on the patient in a single measurement, which could generate a much more appropriate profile of disease status at any given time, a kind of “fingerprint” for the bronchiectasis status of each patient. This tool could help clinicians decide which parameters should be treated in the attempt to improve all aspects of the disease, and how these dimensions may change over time with the treatment selected. In this respect, maximizing the simplicity and specificity of existing tools is of crucial importance. Three examples would be: the simplification of multidimensional scores to assess the severity of the disease; the validation of very simple specific questionnaires that can be administered in clinical practice (a good example would be the COPD Assessment Test [CAT]57); and the identification of specific biomarkers of bronchiectasis that can determine prognosis or response to treatment.
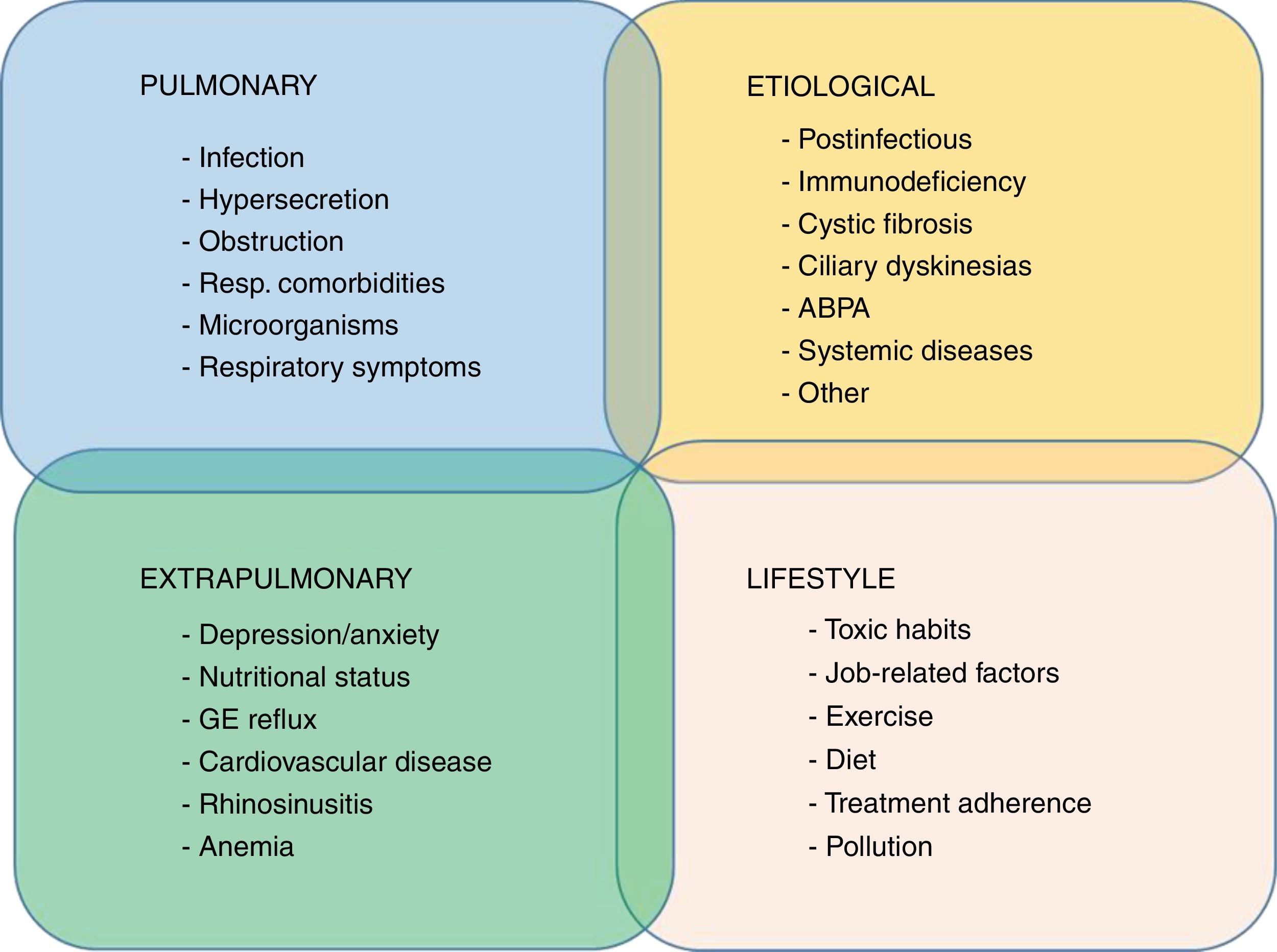
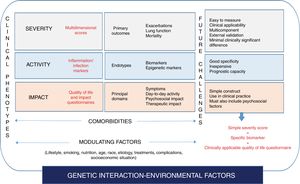
A recent proposal, already implemented in COPD and recently revisited in the setting of bronchiectasis, is the concept of “treatable traits”.58 This is based primarily on the multidimensional treatment of bronchiectasis according to the patient's specific profile, an approach not far removed from personalized medicine.59 A recent publication proposed 4 groups of highly interrelated treatable traits in bronchiectasis: pulmonary changes, extrapulmonary changes, lifestyles, and etiology60 (Fig. 2).
ConclusionBy way of a summary of this review, Fig. 3 attempts to illustrate the heterogeneity and complexity of bronchiectasis (or more accurately, the “bronchiectasis syndrome”) and the position of the different key points (clinical phenotypes, endotypes gravity-activity-disease impact, treatable traits, etc.) within a scheme that would lead to a more holistic understanding of the disease, focusing more on the identification of homogeneous patterns that will lead to the implementation of precision medicine. Our journey on the road toward the understanding of bronchiectasis from this perspective will involve the broad investigation of biomarkers, knowledge of pathophysiological mechanisms, management of the enormous amounts of information (big data and systems biology), and the reduction of costs to make its implementation in clinical practice a feasibility. This century is the century of complexity and precision medicine, “one patient, one treatment, at one time”.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Martínez-García MA, Olveira C, Máiz L, Girón RM, Prados C, de la Rosa D, et al. Las bronquiectasias: una enfermedad compleja y heterogénea. Arch Bronconeumol. 2019;55:427–433.