We have read with great interest the article recently published by Dr. Fernández-Bussy et al.1 about the treatment of airway complications after lung transplantation. The authors describe their experience over the course of 8 years and suggest a treatment algorithm to follow. In our opinion, the study deals with a topic that is currently of great relevance since, first of all, there has been an important growth in lung transplantation activity and, secondly, the possible airway complications that may occur in these patients are not always treated in centers that are specialized in such airway affectations.
As the authors report, given stenosis of the bronchial anastomosis, endoscopic therapy using balloon dilation can be the first option for treatment, requiring the implantation of an endobronquial stent when, after 3 or 4 sessions, definitive results are not obtained. In our group, the most severe stenoses are treated with pneumatic dilation after previously performing radial cuts with electrocauterization in the fibrotic area of the stenosis, followed by the implantation of a stent in selected cases. In previous papers, our group has suggested that the local use of topical mitomycin C, after radial cuts with electrocautery and high-pressure balloon dilation, can avoid this latter measure in a selected subgroup of patients.2,3 The product is an anti-neoplastic agent that inhibits fibroblastic proliferation and has been widely used in locations other than the tracheobronchial tree.4,5
In our experience, since the beginning of the lung transplantation program in our center in October 1993, 335 lung transplantations have been carried out with 537 sutures at risk. A total of 45 airway complications have been detected in 34 patients (10.1% of the total of transplanted patients), the majority of these being in bilateral lung transplantations (60%).
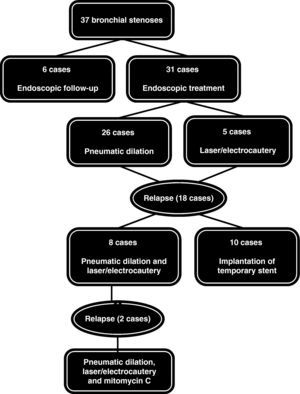
During this period, 37 bronchial stenoses were registered in 28 patients, most of which were circumferential, and in 7 cases they were bilateral. We observed 22 stenoses with affectation only of the segment that encompassed the suture, 10 stenoses with affectation that was distal from the suture and prolonged up until the first- and second-order bronchi, and 5 combined both types. Regarding the therapeutic approach, 6 cases were mild stenoses in which we opted for a series of endoscopic follow-up studies, while 26 required mechanical dilatation and 5 patients were treated with laser-electrocauterization. As for the stenoses that progressed or relapsed after the first endoscopic treatment (n=18), 10 cases required the temporary placement of endoprostheses, while in 8 patients pneumatic dilation was combined with the endobronquial application of laser or electrocautery. In 2 of these cases, we added the local application of mitomycin C given the persistence of endoscopic treatments (Fig. 1). The requirement for this procedure was that at least 3 months had passed since the date of the transplantation. With this interval, the risk is avoided of suture dehiscence with this anti-fibrotic drug. Both cases evolved favorably with stabilization of the bronchial stenosis.
Please cite this article as: Arenas-De Larriva M, et al. Estenosis bronquial postrasplante pulmonar. Arch Bronconeumol. 2011;47:475–6.