To the Editor:
Chylous fistulae as a complication of neck surgery are seen in less than 2% of cases. Chylothorax is even less frequent, and can exceptionally present bilaterally. Since Stuart in 1907 described the first case, we have only found 21 similar cases in the literature.1
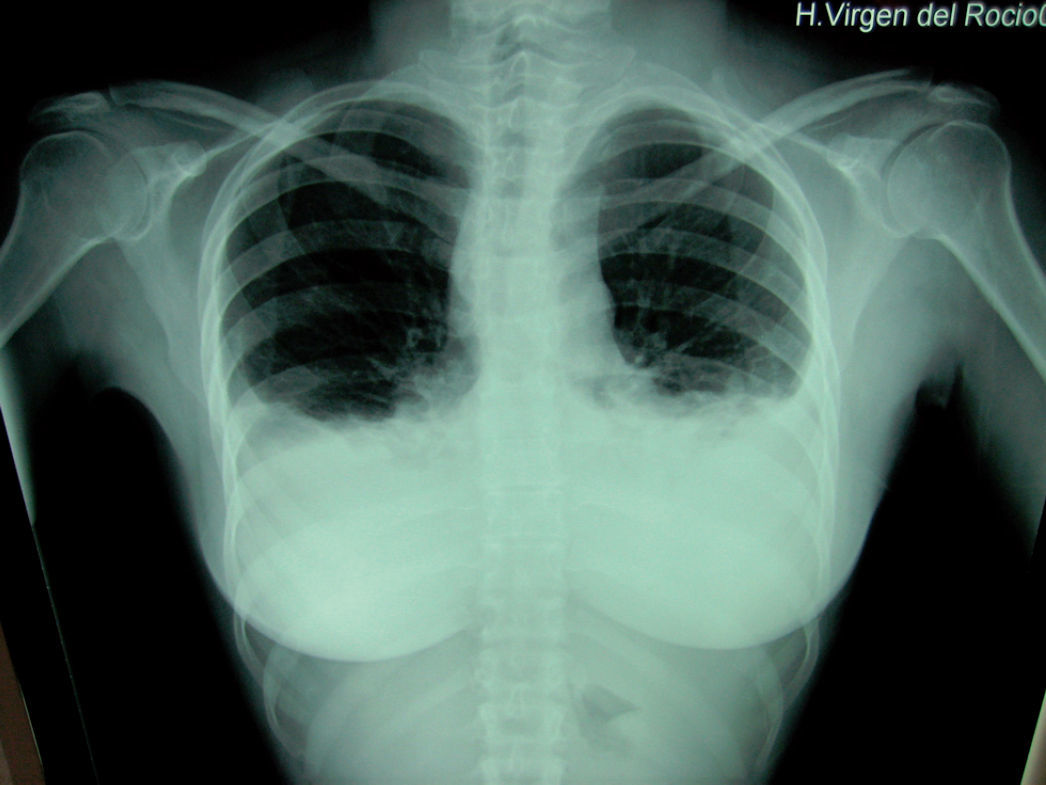
We present a case of a 38 year old woman studied due to the appearance of a palpable cervical 2cm node. On ultrasound it was possible to see a solid left node and a small right node with fine-needle aspiration cytology compatible with papillary thyroid carcinoma. Surgery was performed to carry out a total thyroidectomy with central and left lateral cervical complete lymph node removal. At 72 hours after surgery the patient presented progressive dyspnoea with hypoventilation of both lung bases and clinical symptoms consistent with a pleural effusion which is seen on X-ray (fig. 1). The effusion is greater in the left hemithorax; thoracocentesis is performed obtaining a fluid of chylous aspect. In spite of the placement of a left chest drainage and conservative treatment with total parenteral nutrition, the patient evolved unfavourably with very slight decrease of fluid effusion, therefore we decided to reoperate. On surgery, using a cervical approach, we found a thoracic duct leakage at the level of the brachiocephalic trunk, which we sutured and sealed with biological glue (Bio-glue®), with clinical and radiological resolution of the condition.
In 1875 Cheevers described for the first time a lymphatic fistula of the thoracic duct after neck surgery. It is more frequently seen in left cervical approaches, and its right (25%) or bilateral presentation are exceptional.2
The aetiology of chylothorax may be congenital, obstructive or traumatic. This last, the most frequent, can be either iatrogenic or accidental. The surgical procedures in which chylothorax is most often seen are those that involve the lungs, oesophagus, aortic isthmus or mediastinal tumours, followed by radical cervical dissections, such as the one carried out on the patient we present.3
The physiopathological mechanism of presentation of chylothorax is not clear and two hypotheses are accepted. The first proposes that, through a direct leak, lymph passes into the mediastinum, penetrates the pleura due to an increase in hydrostatic pressure and a secondary inflammatory reaction. The second hypothesis proposes an increase of intraluminal pressure of the thoracic duct after ligature during surgery; during inspiration, negative intrathoracic pressure would cause a lymphatic leakage into the mediastinum without requiring trauma. In our opinion, we think the first hypothesis explains the chylothorax in our patient.4
From the therapeutic point of view, the best approach is preventive, with an appropriate surgical dissection based on complete anatomical knowledge of the area. There are no accepted therapeutic protocols or consensus as to the best approach, and initial conservative treatment is the most accepted. Conservative measures include postural recommendations (rest, elevation of the head of the bed…), compressive bandaging of the surgical area, discontinuation of drainage aspiration, low lipid and low protein diets and parenteral nutrition if improvement is not seen.5
Indications for surgical intervention are persistent fistulae in spite of conservative treatment, significant leakage or associated complications. Some authors propose a limit in quantity or time of drainage, but there is no consensus.6 Although in the literature many substances are reported as being used for sealing or closing the chyle fistula (fibrin, somastotin, tetracycline, etc.), we have not found any reference to biological glues, such as the one we used, which was clinically effective in this case.