Beryllium (Be) is a very light, rigid metal with a high melting point. It is used in alloys, mainly with copper, in the aeronautics industry and in the manufacture of computers, telephones and electronic devices, ceramics, medical devices, dental prostheses, sports equipment, and in jewelry workshops.1 Occupational exposure can cause acute pneumonitis or berylliosis, and more commonly the formation of granulomas caused by a specific CD4 lymphocyte sensitization mechanism, called chronic berylliosis.2,3
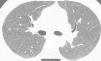
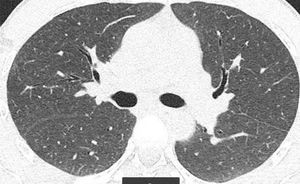
We report the case of a 41-year-old man, former smoker of 10 pack-years, who had worked as a precious stone polisher in a jewelry workshop for 20 years, using compounds containing a Be alloy. He lives in an urban setting, with no exposure to other inhaled substances. He was referred to the respiratory medicine department with a 4-month history of grade 1 dyspnea (MRCm), with no previous respiratory symptoms. Lung function study showed FVC: 4060ml (79%); FEV1: 3330ml (81%); FEV1/FVC: 82%; RV: 2430ml (116%); TLC: 6570ml (89%); DLCO: 26.7ml/min/mmHg (79%) and KCO: 4.84ml/min/mmHg/l (91%). Chest computed tomography identified a faint centroacinar nodular pattern of ground glass density, with poorly defined, bilateral diffuse edges, and slight predominance in the upper lobes. No increase in hilar or mediastinal node size was observed (Fig. 1). Transbronchial biopsy revealed areas of lung tissue with thin interalveolar septa, with no deposits, and foci of inflammatory infiltration with epithelioid cell accumulation, well-defined, without necrosis and with multinucleated cells, not containing foreign material. The bronchoalveolar lavage cell count was as follows: lymphocytes 90%, neutrophils 1%, macrophages 8%, eosinophils 0%, and a CD4/CD8 lymphocyte ratio of 4. Complete microbiological studies of the bronchial aspirate were negative.
Although the data obtained were highly suggestive of sarcoidosis, the possible exposure to Be mentioned in his work history made it necessary to rule out berylliosis. The diagnosis of chronic berylliosis is based on the presence of granulomas in the lung and an immunological reaction of sensitization to Be. To assess this sensitization, the activation response and proliferation of CD4+ lymphocytes to Be in the blood was studied, as follows: mononuclear cells isolated from the patient's peripheral blood were stimulated in vitro with different concentrations of Be (between 10mM and 0.1μM). After 18h of stimulation, the expression of the CD69 activation marker was quantified and IFN-γ production was quantified by ELISPOT. Proliferation was measured by flow cytometry with carboxyfluorescein succinimidyl ester labeling (CFSE 8). Anti-CD3 stimulation was used as a positive control in all functional assays. The patient's cells responded positively to Be stimulation in all assays, in 2 different samples, up to the concentration of 1μM, which confirmed the patient's sensitization to Be.4,5 Cell typing showed a positive DPB1 allele. Mineralogical analysis of the polishing material showed the presence of Be at a concentration of 0.26mg/kg. The final diagnosis was chronic berylliosis. Since the condition was mild, treatment with corticosteroids was not initiated and reduced exposure was recommended. The patient was self-employed and worked in a poorly ventilated area, so he began to use face masks and extractors, and remains stable after 2 years of follow-up.
The presence of pulmonary granulomas requires a differential diagnosis with several entities, both infectious and non-infectious. Of these, sarcoidosis is the most commonly confounded disease. The coexistence of alveolitis, less nodal and systemic involvement, and a history of exposure are the most differentiating elements.6,7 Metal-induced hypersensitivity is type IV (delayed immunity) and is mediated by major histocompatibility complex class II (MHCII). Be activates certain innate and adaptive immunity elements, generating an inadequate immune response in genetically susceptible individuals. The combination of Be with MHCII peptides generates a “neoantigen” that is recognized by CD4+ lymphocytes and, in certain individuals, causes activation and migration of dendritic cells to the lymph nodes, activating sensitized CD4+ lymphocytes which return to the lung where they form granulomas.8 The presence of an HLA-DPB1 allele on chromosome 6 is associated with a high risk of developing Be sensitization and subsequent onset of disease in exposed individuals.9
Progression from sensitization to disease is slow, and depends on the extent of exposure and the genetic elements mentioned. This lymphocyte immune response, together with a description of the type of granulomas involved, are the elements used for the diagnosis of the disease. The granuloma type is very similar to that of sarcoidosis, and for this reason the definitive specific diagnostic test is to detect the activation and proliferation of lymphocytes that show Be sensitization.10 Berylliosis is an occupational respiratory disease that, given the currently widespread use of Be in industry, will continue to produce respiratory disease. Although no data are available, it is safe to assume that, as in other settings,11 exposure to this element in certain industrial sectors in Spain is common, and that given the long latency time, Be-induced respiratory disease will develop that must be treated by pulmonologists trained to differentiate it from other more common entities such as sarcoidosis.12 Our case, probably the first published in Spain, may serve as an excellent reminder to always consider the working environment and the occupation of the patient when collecting their medical history.
Please cite this article as: Martínez González C, Casan Clara P, Prieto Fernández A, Alonso Arias R. Beriliosis: una granulomatosis para no olvidar la importancia de la historia laboral. Arch Bronconeumol. 2020;56:470–471.