To evaluate if the association between the BODE index and deterioration in health-related quality of life is linear. To determine possible associations between the BODE index and health status evaluated by the Saint George's Respiratory Questionnaire (SGRQ) at all levels of disease severity in COPD.
MethodsA cross-sectional study was carried out on 253 patients from two Latin American respiratory centers (Brazil and Chile) with a clinical diagnosis of COPD, based on GOLD criteria. Assessment included the BODE index and the SGRQ questionnaire.
ResultsPatients had a BODE index of 3.1±2.0; FEV1 (%) of 49±19.2; BMI (kg/m2) of 24.7±5.1; 6MWT distance (meters) of 444±96. Significant correlations were found between the BODE index and SGRQ total scores (r=0.5; <0.001), impact (r=0.45; <0.001) and activity (r=0.5; <0.001). From BODE score zero, HRQOL was already compromised in all SGRQ domains. SGRQ scores (total and domain) increased progressively for individual components of the BODE index, with the decrease in airflow limitation (<0.05), BMI (<0.002) and 6MWT (<0.05), and with the increase in the Modified Medical Research Council (MMRC) score (<0.05).
ConclusionThere is an association between health-related quality of life, as assessed by the SGRQ and the BODE index within the entire spectrum of COPD severity. Even in early disease stages and BODE index zero, health-related quality of life is already impaired.
Evaluar si la asociación entre el índice BODE y el deterioro de la calidad relacionada con la salud de la vida es lineal para determinar las posibles asociaciones entre el índice BODE y el estado de salud evaluadas por el Saint George's Respiratory Questionnaire (SGRQ) en todos los niveles de gravedad de la enfermedad en la EPOC.
MétodosUn estudio transversal se llevó a cabo en 253 pacientes de 2 centros latinoamericanos respiratorias (Brasil y Chile) con un diagnóstico clínico de la EPOC, con base en criterios GOLD. La evaluación incluyó el índice BODE y el cuestionario SGRQ.
ResultadosLos pacientes tenían un índice BODE de 3,1±2,0; FEV1 (%) de 49±19,2; IMC (kg/m2) de 24,7±5,1; 6MWT distancia (metros) de 444±96. Se encontraron correlaciones significativas entre las puntuaciones del índice BODE y SGRQ total (r=0,5; <0,001), el impacto (r=0,45; <0,001) y la actividad (r=0,5; <0,001). La calidad de vida relacionada con la salud ya estaba comprometida en todos los dominios del SGRQ a partir de la puntuación cero en BODE. Las puntuaciones del SGRQ, dominio y total, aumentaron progresivamente para los componentes individuales del índice BODE, con la disminución de la limitación del flujo aéreo (<0,05), índice de masa corporal (<0,002) y TC6 (<0,05) y con el aumento de la modificación del Consejo de Investigación Médica (MMRC, Modified Medical Research Council) (<0,05).
ConclusiónExiste una asociación entre la calidad relacionada con la salud de la vida, según la evaluación del SGRQ y el índice BODE dentro de todo el espectro de gravedad de la EPOC. Incluso en bajos estadios de la enfermedad y con el índice BODE en cero, la calidad relacionada con la salud de la vida ya se ha deteriorado.
Chronic obstructive pulmonary disease (COPD) is characterized by partially reversible chronic airway obstruction.1–3 It is associated with an abnormal inflammatory pulmonary response, mainly due to tobacco smoke, and the principal manifestation is dyspnea. The chronic, progressive clinical course of COPD is often aggravated by disease exacerbations that affect lung function and quality of life.4–6
The multidimensional BODE index comprises 4 components: 1 quantifying the grade of lung function impairment (FEV1); 1 capturing patients’ perception of their symptoms (Modified Medical Research Council [MMRC] dyspnea scale; and 2 independent components reflecting the systemic effects of COPD (6-min walk test [6MWT] and body mass index [BMI]). The BODE index has been shown to be a better predictor of survival in COPD patients than FEV1.7,8
Patients with COPD often have poor quality of life due to their symptoms, diminished physical performance and the use of medications.9 Loss of health-related quality of life (HRQoL) is an important marker in COPD patients, since it is a reflection of the impact of the disease on their lives. Quality of life questionnaires have been devised to provide a simple, non-invasive way of measuring patients’ response to treatment. These questionnaires generally feature domains addressing symptoms, functional status, mood and social factors.10–12
Previous studies have shown a weak correlation between FEV1 values and quality of life.7,13,14 The BODE index, used to reflect the systemic involvement of COPD, may correlate better with quality of life questionnaires. Indeed, both the BODE index and quality of life questionnaires include components on symptoms and physical performance.7,15 Medinas-Amorós et al.16 found that the BODE index can predict a loss of quality of life in COPD patients. However, all their subjects presented severe COPD, so their results may not be applicable to the entire disease spectrum. Recently, Iguchi et al.17 showed that the lower the percent of predicted FEV1, the greater the prevalence of depression, but correlation was weak. Using the BODE index, they found that the higher the BODE score, the higher the prevalence of depression.
We hypothesized that there is an association between the BODE index and health status, evaluated using the Saint George Respiratory Questionnaire (SGRQ). The aim of this study was to use data from 2 hospitals in Brazil and Chile to determine if the BODE index is associated with health status, measured by SGRQ, in a population of COPD patients of all levels of severity.
Material and MethodsStudy PopulationThis was a cross-sectional study in patients with typical COPD symptoms and spirometric data consistent with chronic airway obstruction (FEV1/FVC<0.70), according to the definition proposed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD).3 Patients were recruited consecutively in 2 hospital centers, the Federal University of São Paulo (Brazil) and the Universidad Católica de Chile (Santiago de Chile). Airway obstruction severity was evaluated from the percent of predicted FEV1, according to GOLD criteria: mild, FEV1≥80% predicted value; moderate, FEV1≥50% and <80% predicted value; severe: FEV1≥30% and <50% predicted value; very severe: FEV1<30% predicted value.3 Eligibility criteria were as follows: clinical stability, i.e., no increase in wheezing, cough, dyspnea or sputum production during the previous 30 days; no use of systemic corticosteroids or antibiotics during the previous month; and signed informed consent statement.16 Exclusion criteria were as follows: any disease preventing the patient from performing the tests appropriately; current smoker or ex-smoker for less than 6 months (in general, COPD patients seen in our outpatient clinic who smoke are monitored regularly in the clinic and referred to our smoking cessation clinic); and any unstable comorbidities, such as lung cancer, diabetes, heart failure, asthma, etc. The study was approved by the ethics committees of both universities and all patients signed informed consent statements. Internal Research Committee: Federal University of São Paulo, CEP 1209/05, and Universidad Católica de Chile, CE 0168/07.
AssessmentsWeight was measured on calibrated scales, and height with a stadiometer. BMI was calculated by dividing weight in kilograms by height in meters squared. BMI values were categorized as ≤21 and >21, as described by Celli et al.7
Spirometry was performed using a portable, battery-powered, ultrasonic transit time spirometer (Easy-One; Model 2001 Diagnostic Spirometer, NDD Medical Technologies, Zurich, Switzerland), calibrated daily with a 3l syringe. Forced expiration maneuvers were performed with the patient in a sitting position, as described by the American Thoracic Society (ATS), before and 15min after inhalation of 400μg salbutamol via a metered dose inhaler. Predicted values were calculated according to ATS recommendations.18
Dyspnea was assessed according to the MMRC scale.19 For the six-minute walk test (6MWT), the patient walked along a straight, 25-m long corridor, receiving a standard verbal stimulus every minute, according to ATS recommendations.20 Two walk tests were performed on the same day, the second after a 45-min interval, when physiological variables had returned to baseline. The best of the 2 walks was used for the analysis.
BODE index was calculated after all the necessary variables had been obtained (BMI, airway obstruction, dyspnea and exercise capacity). Each variable was categorized on a scale of 0 to 3, except for BMI, which was taken as a dichotomous variable (0 or 1). The sum of the variables was used to obtain a BODE score of 0–10.7
Health status was evaluated with the administration of the specific 76-item SGRQ with 3 components: symptoms, activity and impact of disease on activities of daily living. SGRQ was self-administered by each patient. Items were scored from 0 (best state of health) to 100 (worst state of health). Weighted scores from each dimension were summed. Subscale and total quality of life scores from the summed weights were divided by the sum of maximum possible weights for each dimension. SGRQ has been previously validated in Brazilian Portuguese10 and Spanish.21
Statistical AnalysisContinuous variables are presented as mean±standard deviation (SD). Discrete variables are expressed in absolute numbers and percentages of the total. The Kolmogorov–Smirnov normality test was used to evaluate the variable data distribution. Patients from Brazil and Chile were compared using the Student's t-test for independent samples and a χ2-test. Additional analyses were performed using the Levene test for equality of variances and analysis of variance (ANOVA) when necessary. Tukey's post hoc test was used for multiple comparisons. Pearson's coefficient comparison was used to determine linear associations between numerical variables. Statistical significance was established at P<.05. Sample size was calculated using the lowest correlation coefficient obtained for α=0.01 and β=0.20, determining the inclusion of at least 67 subjects.22
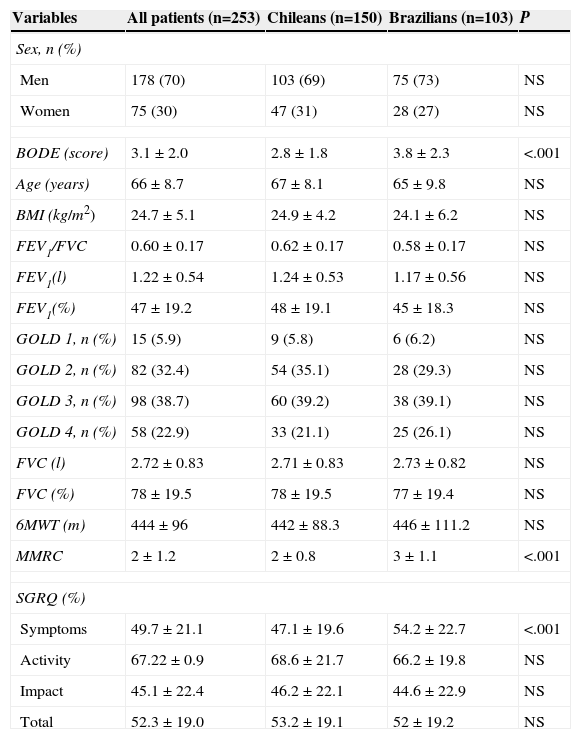
ResultsA total of 253 COPD patients (103 Brazilians and 150 Chileans) were included; 70% were men; mean age 66±8.7 years; mean FEV1/FVC 0.60±0.17; mean FEV1 47±19.2% predicted, and mean FVC 78±19.5% predicted. The mean BMI was 24.7±5.1kg/m2, so patients were classified as having a normal nutritional status. Mean 6MWT distance was 444±96.0m. Mean total BODE index score was 3.1±2.0. Baseline patient characteristics in both centers are shown in Table 1: no overall differences were observed.
Characteristics of all Patients Pooled, and by Center.
| Variables | All patients (n=253) | Chileans (n=150) | Brazilians (n=103) | P |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Men | 178 (70) | 103 (69) | 75 (73) | NS |
| Women | 75 (30) | 47 (31) | 28 (27) | NS |
| BODE (score) | 3.1±2.0 | 2.8±1.8 | 3.8±2.3 | <.001 |
| Age (years) | 66±8.7 | 67±8.1 | 65±9.8 | NS |
| BMI (kg/m2) | 24.7±5.1 | 24.9±4.2 | 24.1±6.2 | NS |
| FEV1/FVC | 0.60±0.17 | 0.62±0.17 | 0.58±0.17 | NS |
| FEV1(l) | 1.22±0.54 | 1.24±0.53 | 1.17±0.56 | NS |
| FEV1(%) | 47±19.2 | 48±19.1 | 45±18.3 | NS |
| GOLD 1, n (%) | 15 (5.9) | 9 (5.8) | 6 (6.2) | NS |
| GOLD 2, n (%) | 82 (32.4) | 54 (35.1) | 28 (29.3) | NS |
| GOLD 3, n (%) | 98 (38.7) | 60 (39.2) | 38 (39.1) | NS |
| GOLD 4, n (%) | 58 (22.9) | 33 (21.1) | 25 (26.1) | NS |
| FVC (l) | 2.72±0.83 | 2.71±0.83 | 2.73±0.82 | NS |
| FVC (%) | 78±19.5 | 78±19.5 | 77±19.4 | NS |
| 6MWT (m) | 444±96 | 442±88.3 | 446±111.2 | NS |
| MMRC | 2±1.2 | 2±0.8 | 3±1.1 | <.001 |
| SGRQ (%) | ||||
| Symptoms | 49.7±21.1 | 47.1±19.6 | 54.2±22.7 | <.001 |
| Activity | 67.22±0.9 | 68.6±21.7 | 66.2±19.8 | NS |
| Impact | 45.1±22.4 | 46.2±22.1 | 44.6±22.9 | NS |
| Total | 52.3±19.0 | 53.2±19.1 | 52±19.2 | NS |
6MWT (m): 6-min walking test (meters); BODE index: body mass index, forced expiratory volume in 1 second, Modified Medical Research Council and 6-min walking test, in meters; BMI: body mass index; FEV1 (%): forced expiratory volume in 1s, % predicted; FEV1 (l): forced expiratory volume in 1s (liters); FEV1/FVC: forced expiratory volume in 1s/forced vital capacity ratio; FVC (%): forced vital capacity, % predicted; FVC (l): forced vital capacity (liters); MMRC: Modified Medical Research Council.
Data are presented as mean±SD or number (%).
Significant correlations were observed between the BODE index and the SGRQ components: symptoms (r=0.28; <0.001), activity (r=0.52; <0.001), impact (r=0.45; <0.001) and total score (r=0.50; <0.001).
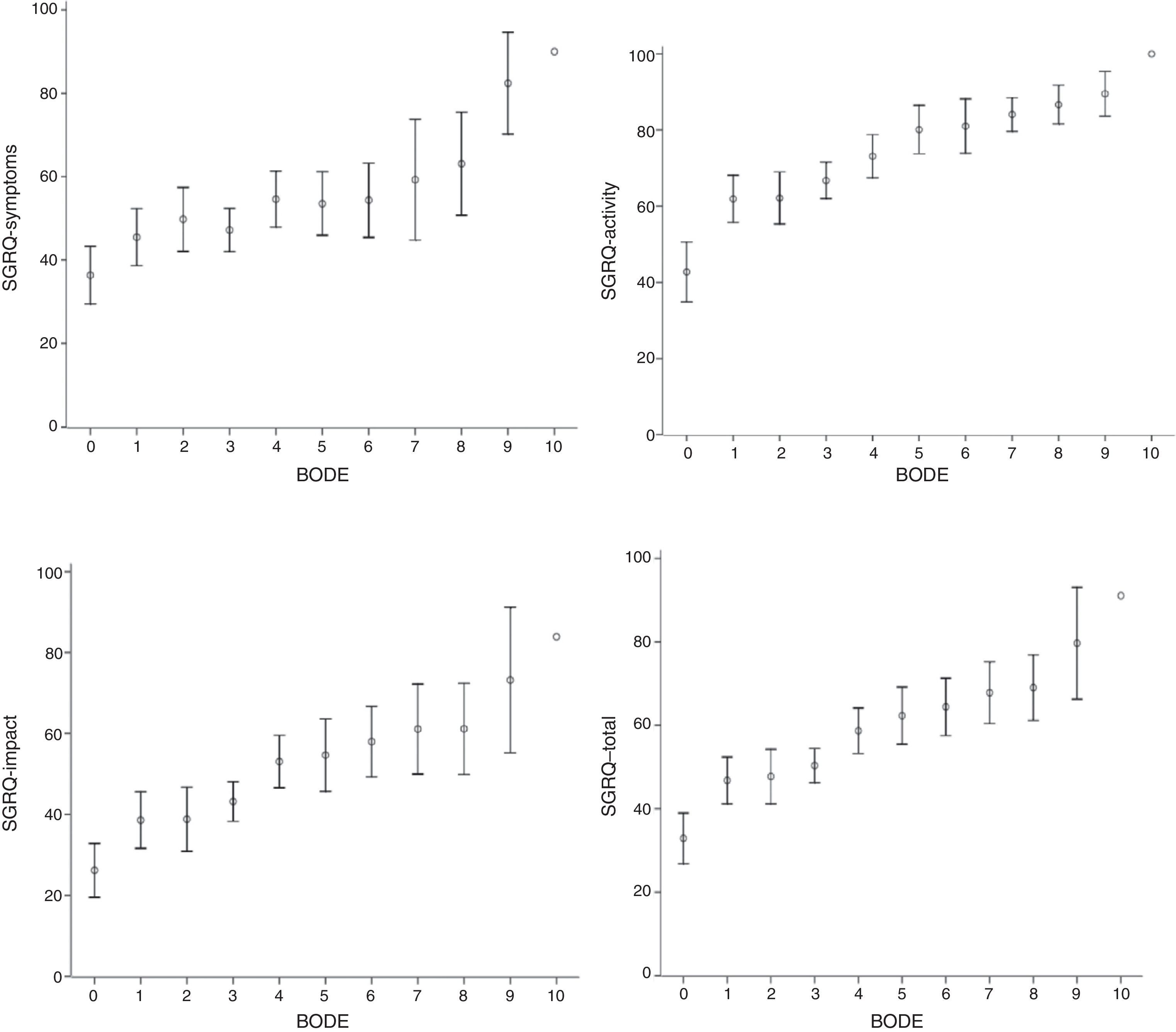
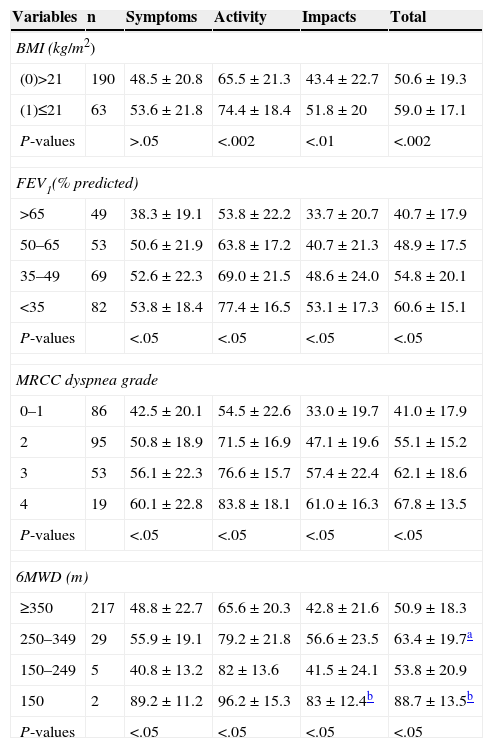
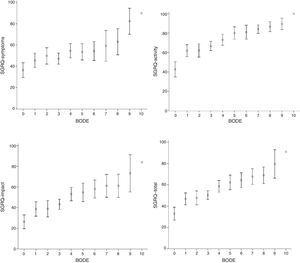
SGRQ component scores (symptoms, activity, impact and total) compared to individual BODE scores, stratified for BMI (kg/m2) (<0.002), airway obstruction (FEV1) (<0.05), dyspnea (MMRC) (<0.05) and exercise (6MWT) (<0.05) are shown in Table 2. The evolution of all components was very similar, and even with a BODE score of 0, HRQoL was greater than 10%, suggesting that quality of life deteriorates in parallel with increasing BODE score. The relationship between these associations, more clearly demonstrated in Fig. 1, was not linear.
Saint George's Respiratory Questionnaire (SGRQ) Mean Scores Compared to Body Mass Index (BMI), Forced Expiratory Volume in 1s (FEV1), Modified Medical Research Council (MMRC) and 6-Min Walk Test (6MWT), by BODE Strata.
| Variables | n | Symptoms | Activity | Impacts | Total |
|---|---|---|---|---|---|
| BMI (kg/m2) | |||||
| (0)>21 | 190 | 48.5±20.8 | 65.5±21.3 | 43.4±22.7 | 50.6±19.3 |
| (1)≤21 | 63 | 53.6±21.8 | 74.4±18.4 | 51.8±20 | 59.0±17.1 |
| P-values | >.05 | <.002 | <.01 | <.002 | |
| FEV1(% predicted) | |||||
| >65 | 49 | 38.3±19.1 | 53.8±22.2 | 33.7±20.7 | 40.7±17.9 |
| 50–65 | 53 | 50.6±21.9 | 63.8±17.2 | 40.7±21.3 | 48.9±17.5 |
| 35–49 | 69 | 52.6±22.3 | 69.0±21.5 | 48.6±24.0 | 54.8±20.1 |
| <35 | 82 | 53.8±18.4 | 77.4±16.5 | 53.1±17.3 | 60.6±15.1 |
| P-values | <.05 | <.05 | <.05 | <.05 | |
| MRCC dyspnea grade | |||||
| 0–1 | 86 | 42.5±20.1 | 54.5±22.6 | 33.0±19.7 | 41.0±17.9 |
| 2 | 95 | 50.8±18.9 | 71.5±16.9 | 47.1±19.6 | 55.1±15.2 |
| 3 | 53 | 56.1±22.3 | 76.6±15.7 | 57.4±22.4 | 62.1±18.6 |
| 4 | 19 | 60.1±22.8 | 83.8±18.1 | 61.0±16.3 | 67.8±13.5 |
| P-values | <.05 | <.05 | <.05 | <.05 | |
| 6MWD (m) | |||||
| ≥350 | 217 | 48.8±22.7 | 65.6±20.3 | 42.8±21.6 | 50.9±18.3 |
| 250–349 | 29 | 55.9±19.1 | 79.2±21.8 | 56.6±23.5 | 63.4±19.7a |
| 150–249 | 5 | 40.8±13.2 | 82±13.6 | 41.5±24.1 | 53.8±20.9 |
| 150 | 2 | 89.2±11.2 | 96.2±15.3 | 83±12.4b | 88.7±13.5b |
| P-values | <.05 | <.05 | <.05 | <.05 | |
Data are presented as mean±SD. SGRQ compared to BMI categories of BODE index, according to t-test for independent samples. SGRQ compared to FEV1, MMRC, 6MWT and BODE index categories, according to ANOVA with Tukey's post hoc testing.
Saint George's Respiratory Questionnaire (SGRQ) component and total scores compared to BODE index. Data are presented as mean (±SD). Vertical arrows indicate change in SGRQ score. All SGRQ components increase as the BODE index rises from 0 to 1, after which they stabilize until BODE score reaches 3, at which point another clear increase is seen in SGRQ. As before, health-related quality of life (HRQoL) was relatively stable when BODE scores increased from 4 to 7.
Fig. 1 shows that all SGRQ components were affected, even when BODE score was 0. When BODE score shifted from 0 to 1, all components rose sharply by 14 points. Between this score and a BODE score of 3, SGRQ was stable, but subsequently there was another steep increase of 8 points. At BODE scores of between 4 and 7, SGRQ was relatively stable.
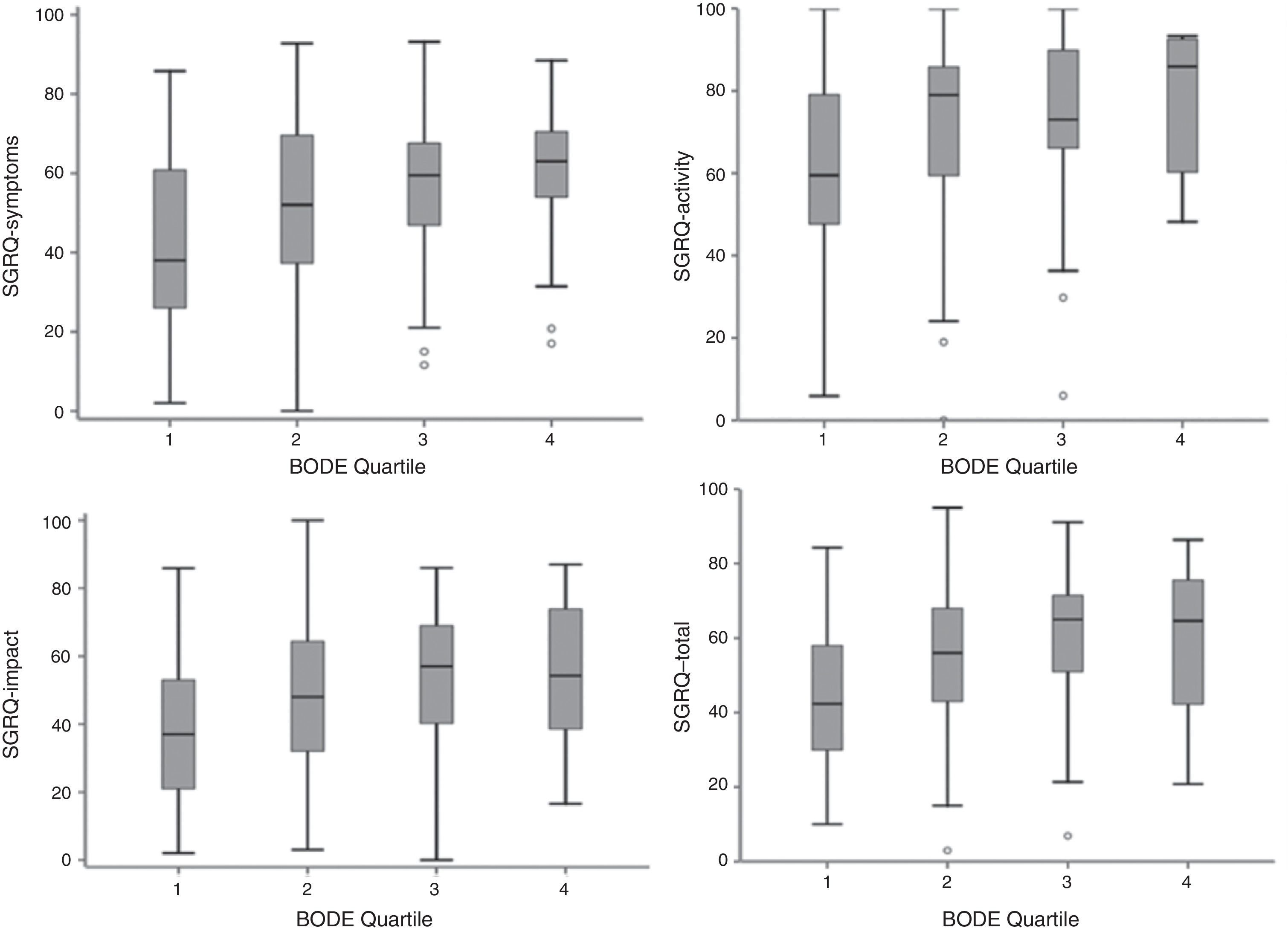
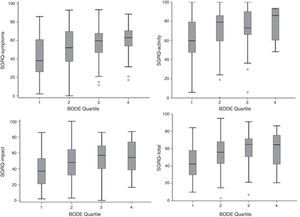
Component and total SGRQ scores compared to the BODE index quartile are shown in Fig. 2. The plot is similar to that of Fig. 1, with a poorer quality life being reflected by a higher BODE index quartile.
DiscussionFEV1 has been used for many years as the only predictive parameter for mortality in COPD patients.7,8,23 However, the correlation between FEV1 and symptoms, quality of life, frequency of exacerbations and exercise intolerance is weak.24,25 Moreover, while FEV1 is essential for the diagnosis and quantification of respiratory impairment caused by COPD, it does not give an accurate picture of the systemic manifestations of the disease. The BODE index was developed as a multidimensional index, and associates FEV1 with a parameter for perception of symptoms (MMRC dyspnea scale) and 2 independent components reflecting the systemic consequences of COPD (6MWT distance and BMI). Celli et al.7 showed that this multidimensional index was clearly superior to FEV1 as a predictor of mortality in COPD patients.
Medinas-Amorós et al.16 found an association between HRQoL and the BODE index in patients with severe COPD. However, as patients with mild to moderate COPD were not evaluated in this study, the results may not be applicable to other disease stages. In another study, the same group evaluated the association between HRQoL and COPD severity, according to GOLD staging, and the BODE index in a small series of 64 patients. They found a closer relationship between HRQoL and COPD severity evaluated with the BODE index than with GOLD staging.26 Araujo et al.27 correlated the BODE index with HRQoL in COPD patients in a small series of 42 patients, distributed in 2 groups according to disease severity: FEV1≥50% and FEV1<50%, They concluded that the BODE index score was closely related with all SGRQ components, but only in the subgroup of patients with FEV1<50%.
The most interesting finding of our study is that a BODE score of 0 already compromised HRQoL in all SGRQ components. It should also be mentioned that this relationship is not linear, as can be seen in Figs. 1 and 2. A sharp increase is seen in all SGRQ components when BODE score increased from 0 to 1, after which they stabilize until BODE score reaches 3, at which point another sharp increase is seen in SGRQ. As before, HRQoL was relatively stable when BODE scores increased from 4 to 7. This lack of linearity in the correlation between the BODE score and HRQoL is very interesting, yet puzzling. Diminishing HRQoL in COPD patients has already been shown to have no linear relationship with increasing disease severity.28 Our data suggest that patients can adapt to their mounting limitations up to a certain critical level. When these critical levels are reached, HRQoL deteriorates again. These results appear to complement the work of Medinas-Amorós et al.,26 by indicating an association between SGRQ and the BODE index in COPD patients at all disease stages.
In this study, the physical activity component (6MWT) showed the closest associations between health status and the BODE index, which reflects in part the systemic nature of COPD (r=0.52; <0.001). Walking is an important aspect of patients’ daily activities and depends on respiratory function as well as cardiopulmonary status, nutrition, and peripheral muscle strength.29,30 These results clearly show that functional limitation in COPD patients affects their quality of life.31 Watz et al32 previously found that patients with moderate COPD (GOLD stage 2) with a score of 1 on the BODE index have limited physical activity. This appears to suggest that COPD patients should be referred for rehabilitation, even in the early stages of the disease.
Recent Spanish COPD guidelines (GesEPOC)33 recommend directing the type of treatment by phenotype and the intensity of treatment by its severity. Evaluation of severity should be based on the BODE multidimensional index, or BODEex, that classifies the disease into 4 stages: mild, moderate, severe and very severe. According to this system, our patients should be classified as moderate or stage II, since the mean BODE index score was 3, a level that impacts severely on quality of life. The GOLD document also recognizes that FEV1 is an essential parameter, but insufficient in itself for classifying a patient. Thus, these guidelines recommend also determining dyspnea (Medical Research Council), exacerbations and hospitalizations, quality of life (COPD Assessment Test) and clinical status (COPD Clinical Questionnaire).34
SGRQ is a specific respiratory questionnaire. It comprises 3 components that go some way toward expressing the systemic status of the patient (activity and impact components). However, the responses to SGRQ questions reflect the patient's mood at the time of the evaluation. As such, they are susceptible to the effect of the patient's mood at that time, and may be rather subjective.34 In contrast, the 4 BODE index dimensions are quite objective, since they are based on direct measurements.7 The satisfactory association found between both instruments suggests that one complements the other, and that both may be used for improving the evaluation of COPD patients. Our results are supported by Lin et al.,35 who concluded that the BODE index may be useful as a sensitive instrument for evaluating patients’ quality of life status and for monitoring disease progression in patients with stable COPD. Recently, Marin et al.36 found that both the BODE index and the SGRQ predicted mortality and provided complementary information in the evaluation of COPD patients. Jones et al.37 observed marked HRQoL impairment even in the most mild forms of the disease, and little difference in the degree of impairment between patients in GOLD stage I and II. HRQoL impairment varied widely within each GOLD stage of severity, and these authors conclude that HRQoL must be a routine part of the evaluation and monitoring of COPD patients in primary care.
The advantage of some of these instruments is that, if used in the baseline evaluation, they can provide specific information for guiding clinical practice; for example, a BODE index score of 0 can help physicians identify individuals needing referral to special rehabilitation care.
Cote and Celli38 found that the BODE score of COPD patients following a rehabilitation program fell, but they did not report on the effects that rehabilitation may have had on quality of life. Quality of life questionnaires are useful for describing patients’ perception of their disease and its impact. We have shown a close association between quality of life and the BODE index, suggesting that the quality of life of Cote and Celli's patients may also have improved and the risk of mortality diminished. However, no studies have so far shown an improvement in quality of life within the same BODE stage.
Although our study was well designed and the sample size was large, it is limited by the small number of women in the study population, reflecting the fact that the number of women seen in our clinics is lower than the number of men. However, this appears to be a general pattern, and has been seen in several COPD and pulmonary rehabilitation studies.
In conclusion, this study has shown an association between HRQoL (evaluated by SGRQ) and the BODE index for patients across the spectrum of COPD severity. The activity component of the SGRQ showed the closest association with the BODE score. COPD patients with a BODE score of 0 already have poor quality of life, and this deteriorates in a stepwise fashion. This suggests that these patients gradually adapt to their situation until they reach a new stage of impairment. To our knowledge, this is the first study to evaluate the relationship between the BODE index and health status determined using a specific respiratory questionnaire (SGRQ) in a large series of COPD patients representing all stages of severity.
Conflict of InterestThe authors state that they have no conflict of interests.
Please cite this article as: Nonato NL, Díaz O, Nascimento OA, Dreyse J, Jardim JR, Lisboa C. Comportamiento de la calidad de vida (SGRQ) en pacientes con EPOC según las puntuaciones BODE. Arch Bronconeumol. 2015;51:315–321.