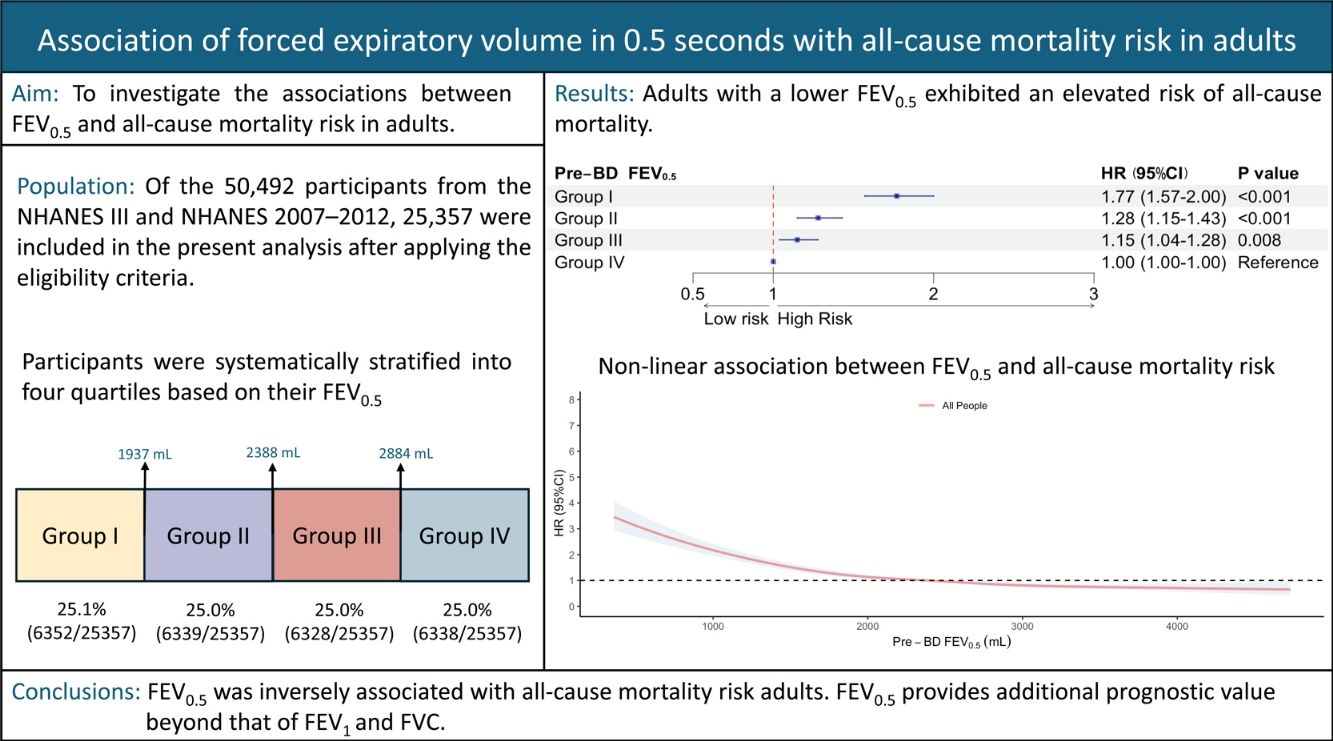
Previous studies have proposed forced expiratory volume in 0.5s (FEV0.5) to determine health outcomes in infants and young children, but few studies exist in adults. This study aims to investigate the associations between FEV0.5 and all-cause mortality in adults.
MethodsParticipants were enrolled from the National Health and Nutrition Examination Survey (NHANES) (1988–1994 [NHANES III] and 2007–2012 cycles). Participants aged≥20 years, not pregnant with qualifying prebronchodilator FEV0.5 data, acceptable spirometry, complete body measurements, and follow-up data for mortality were included. The association between FEV0.5 and all-cause mortality risk was evaluated by multivariable Cox regression. Restricted cubic spline analysis was used to evaluate the non-linear relationship between FEV0.5 and all-cause mortality. Subgroup analyses were conducted with stratification by sex, age, body mass index, smoking status, and race.
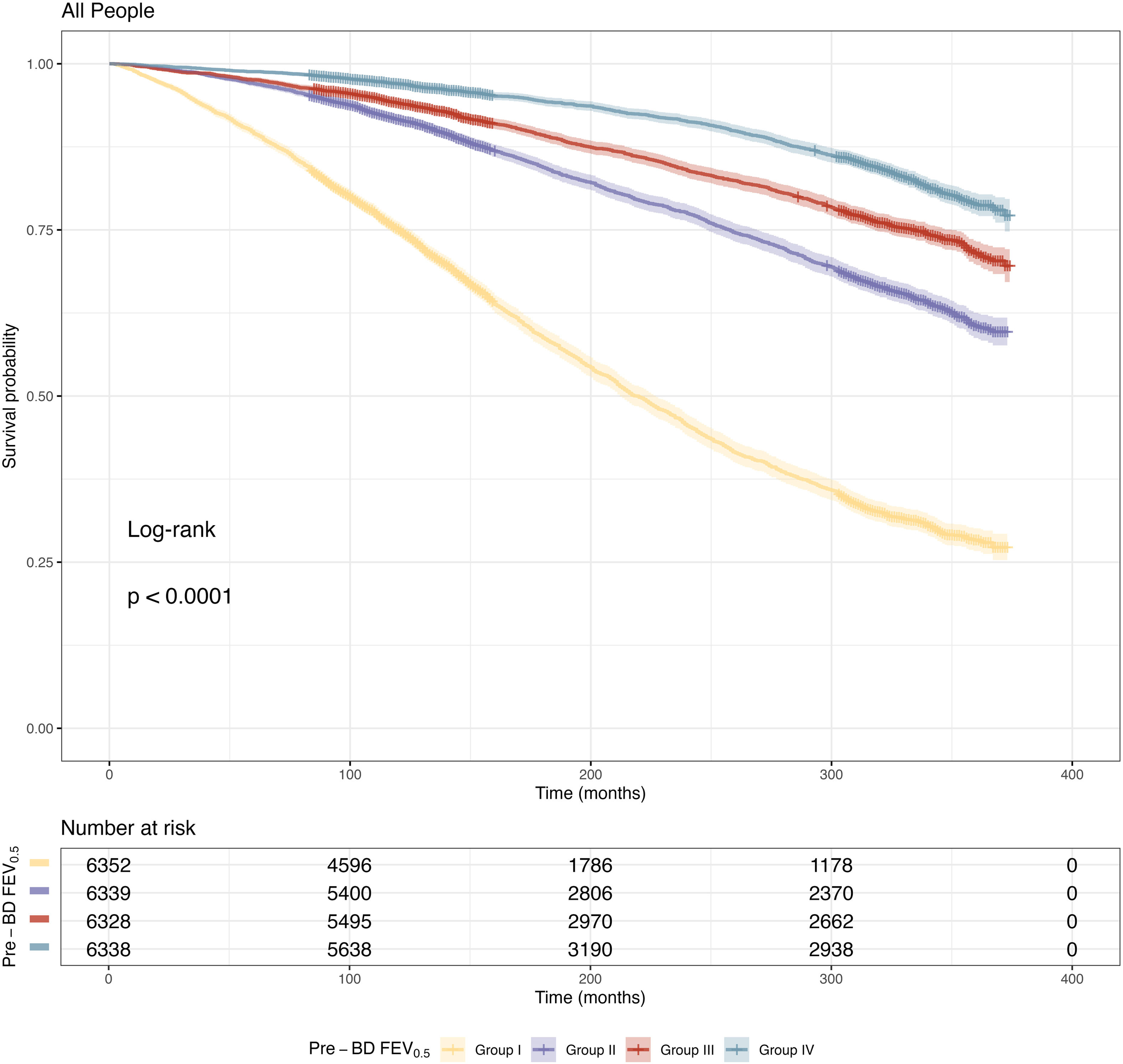
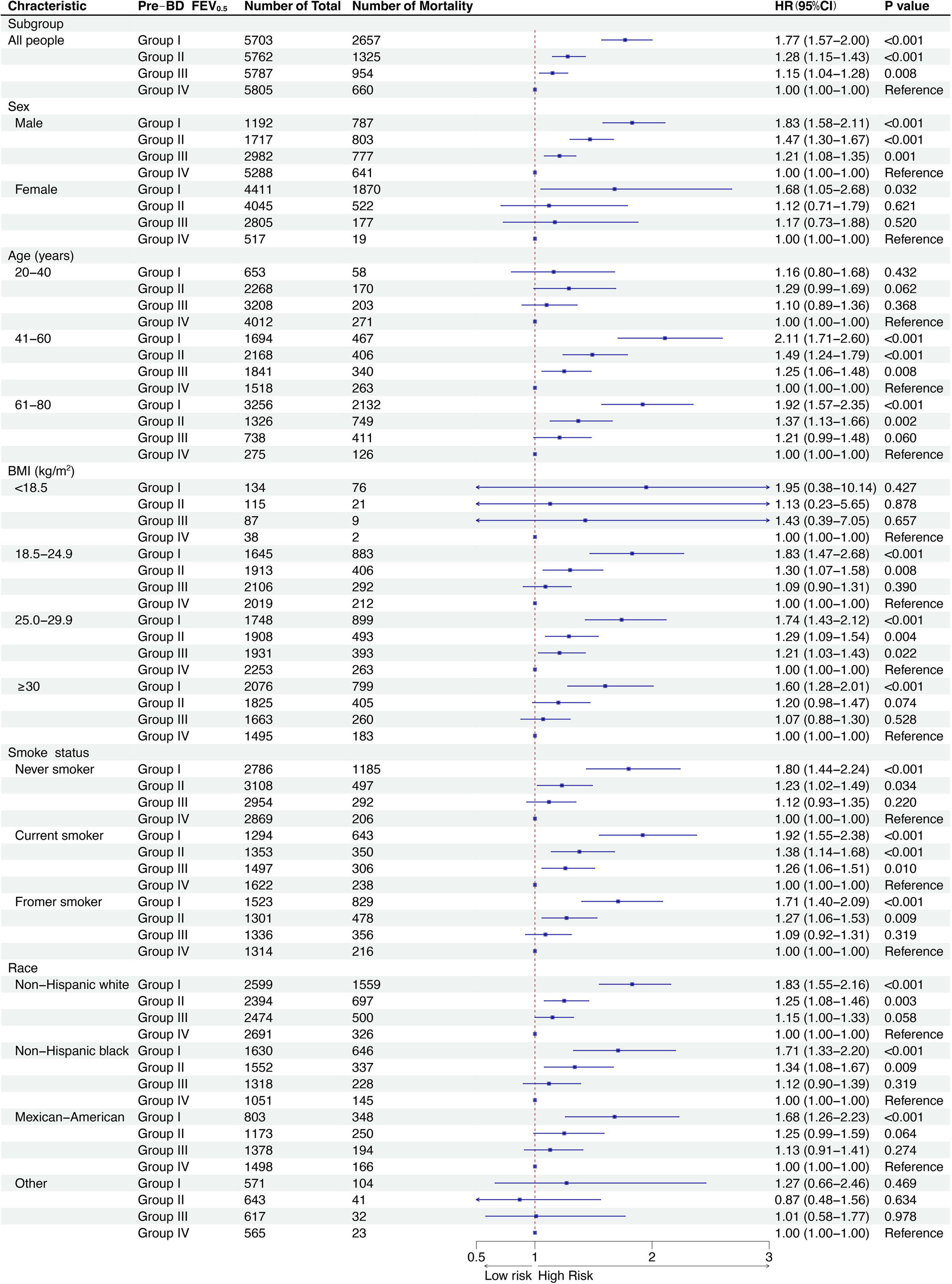
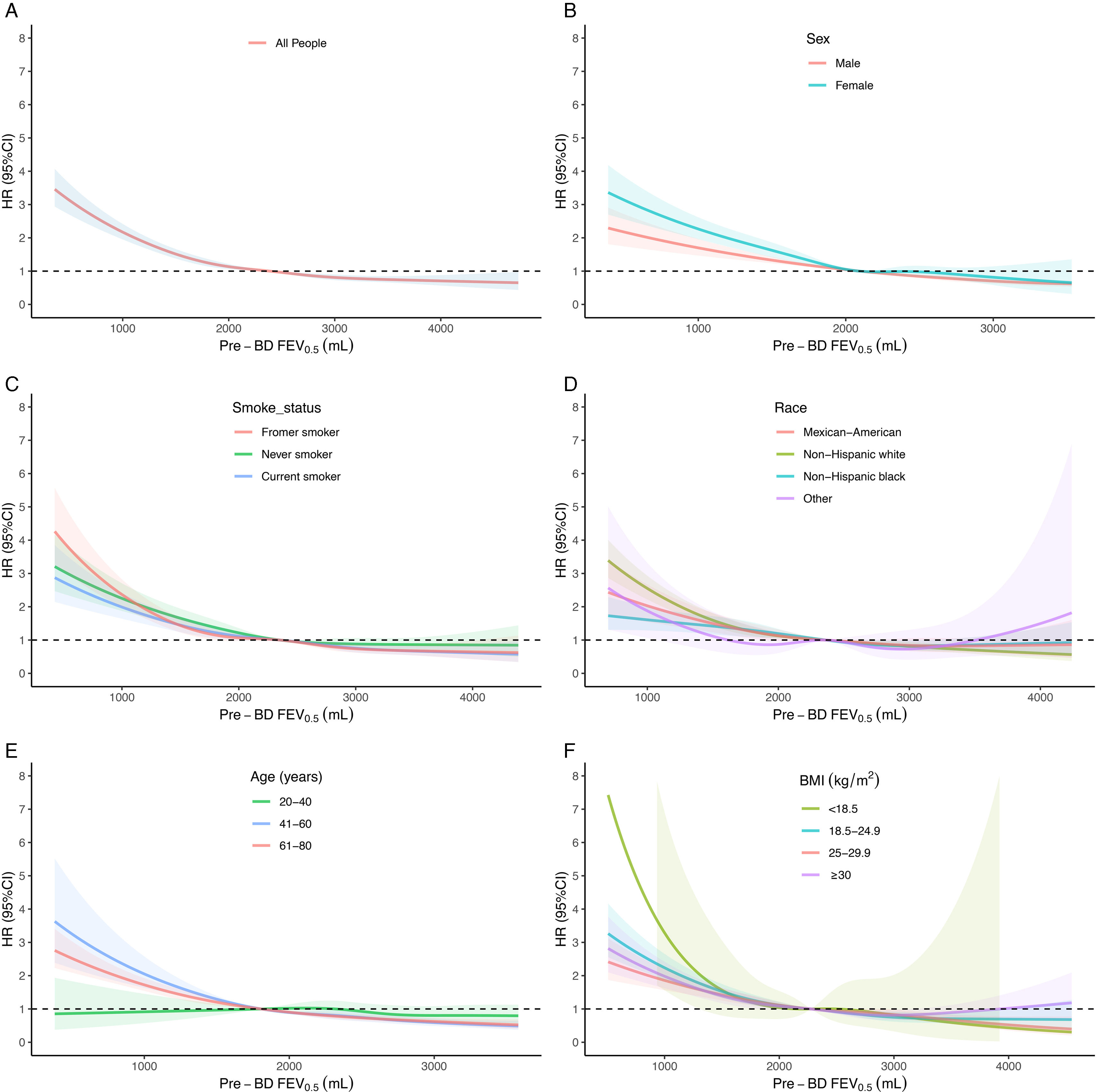
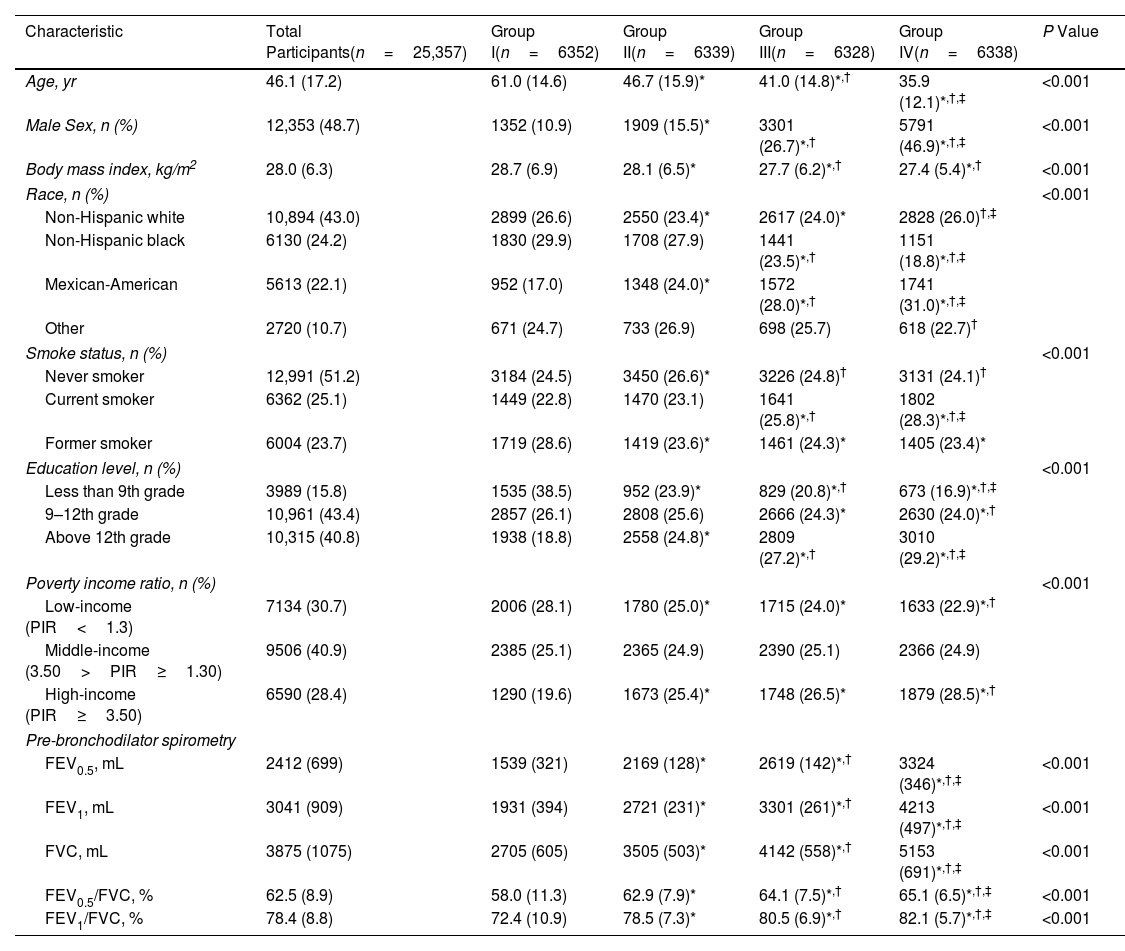
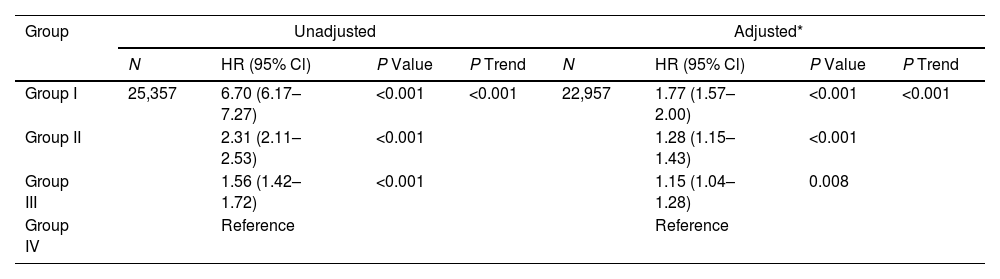
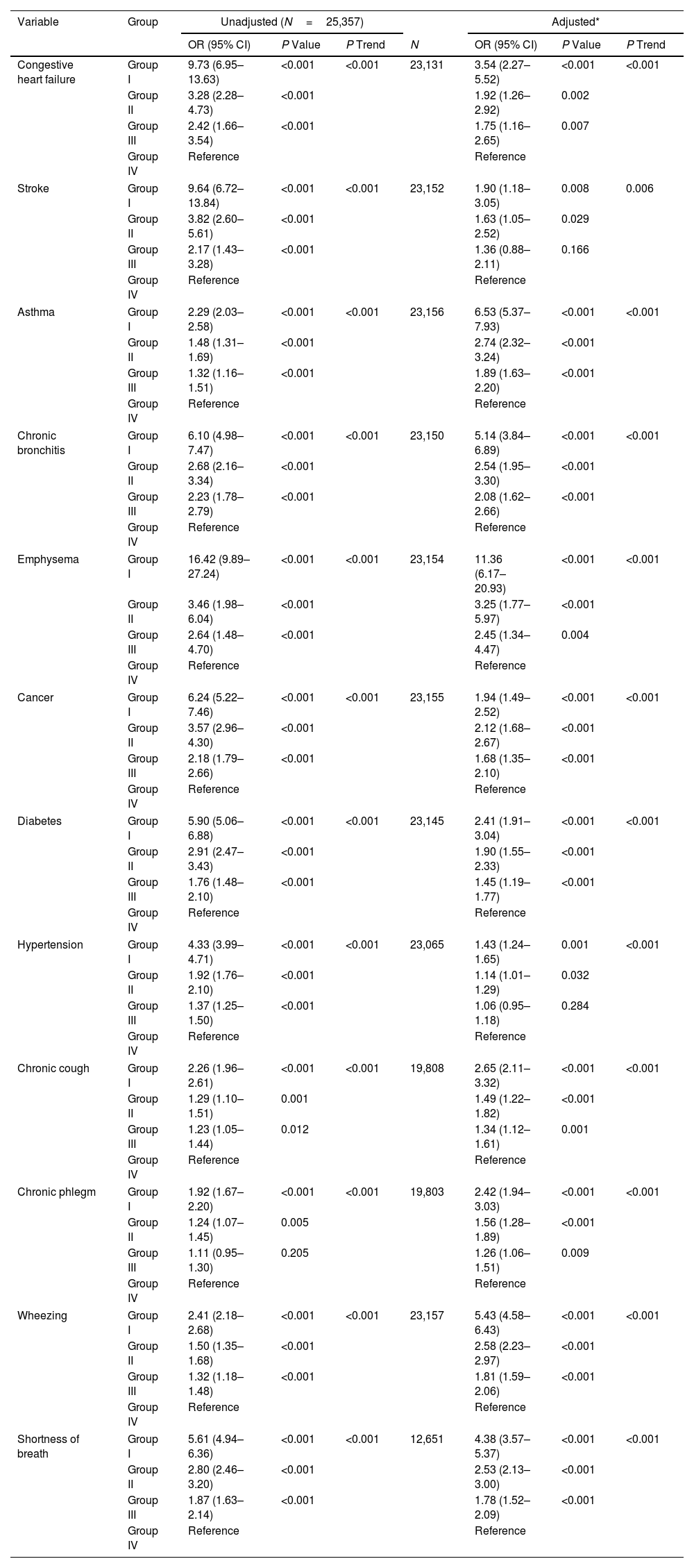
ResultsOverall, 25,357 individuals were included, with a median follow-up of 308 months. The mean±standard deviation age was 46.1±7.2 years, and the mean prebronchodilator FEV0.5 was 2412±699mL. A reduction in FEV0.5 was associated with an increased all-cause mortality risk. A non-linear relationship was observed between FEV0.5 and all-cause mortality risk. The results were maintained in subgroups analyses.
ConclusionFEV0.5 was inversely associated with all-cause mortality risk in adults, indicating its potential for monitoring respiratory health.