To determine the prevalence of arterial stump thrombosis (AST) after pulmonary resection surgery for lung cancer and to describe subsequent radiological follow-up and treatment.
Material and methodsObservational, descriptive study of AST detected by computerized tomography angiography (CT) using intravenous contrast. Clinical and radiological variables were compared and a survival analysis using Kaplan–Meier curves was performed after dividing patients into 3 groups: patients with AST, patients with pulmonary embolism (PE), and patients without AST or PE.
ResultsNine cases of AST were detected after a total of 473 surgeries (1.9%), 6 of them in right-sided surgeries (67% of AST cases). Median time to detection after surgery was 11.3 months (interquartile range 2.7–42.2 months), and range 67.5 months (1.4–69.0 months). Statistically significant differences were found only in the number of CTs performed in AST patients compared to those without AST or PE, and in tumor recurrence in PE patients compared to the other 2 groups. No differences were found in baseline or oncological characteristics, nor in the survival analysis.
ConclusionsIn this series, AST prevalence was low and tended to occur in right-sided surgeries. Detection over time was variable, and unrelated to risk factors previous to surgery, histopathology, and tumor stage or recurrence. AST had no impact on patient survival.
Determinar la prevalencia de trombosis de muñón arterial (TMA) en cirugías de resección pulmonar por carcinoma broncogénico, y describir su evolución radiológica y tratamiento.
Material y métodosEstudio observacional y retrospectivo de casos de TMA detectados mediante angiotomografías con contraste intravenoso (TAC). La comparación de variables clínicas, radiológicas, y el análisis de supervivencia mediante curvas de Kaplan-Meier, se realizó planteando 3 grupos: pacientes con TMA, pacientes con tromboembolismo pulmonar (TEP) y pacientes sin TMA ni TEP.
ResultadosSe detectaron 9 TMA en 473 cirugías (1,9%), 6 de ellas en el lado derecho (67% de las TMA), con una mediana de tiempo de detección desde la cirugía de 11,3 meses (rango intercuartílico 2,7-42,2 meses). Salvo el número de TAC en pacientes con TMA comparados con el grupo sin TEP ni TMA, y la recidiva tumoral en pacientes con TEP en comparación con los restantes 2 grupos, no se encontraron diferencias estadísticamente significativas en las características basales ni en las oncológicas. Igualmente no se encontraron diferencias en el análisis de supervivencia.
ConclusionesEn nuestra serie, la TMA fue una patología infrecuente que tendió a localizarse en las cirugías del lado derecho, y cuya detección a lo largo del tiempo fue variable. No se asoció a factores de riesgo previos a la cirugía ni tuvo predisposición en relación con la estirpe histológica, estadificación oncológica o recidiva tumoral.
Arterial stump thrombosis (AST) in pulmonary resection surgery has been described, but this condition, particularly the prognostic implications and the correct therapeutic approach, has not been studied in depth. Since the first 2 cases of AST in pneumonectomies were described in 1966,1 only 2 studies,2,3 small series and isolated case reports have been published in the literature, all retrospective. Although in the past little importance has been given to this finding, in recent years, the generalized use of imaging techniques in follow-up, and the improved resolution of these procedures have led to a growing number of reports of cases with associated complications.4–11
The main objectives of our study were to determine the prevalence of AST in oncological lung resection surgery, to identify risk factors present before the intervention and those caused by the procedure itself, and to describe subsequent progress in terms of radiological follow-up and treatment. The secondary objective was to determine clinical, oncological and prognostic differences between patients with AST, patients who did not have any type of thrombosis, and those who had pulmonary thromboembolism (PTE).
Materials and MethodsDesignObservational retrospective study of a historical cohort of patients undergoing pulmonary resection for lung cancer. Following the indications of the local Ethics Committee, the observational, retrospective design of this study precluded the need for informed consent. The report was drafted in accordance with the STROBE statement guidelines.12
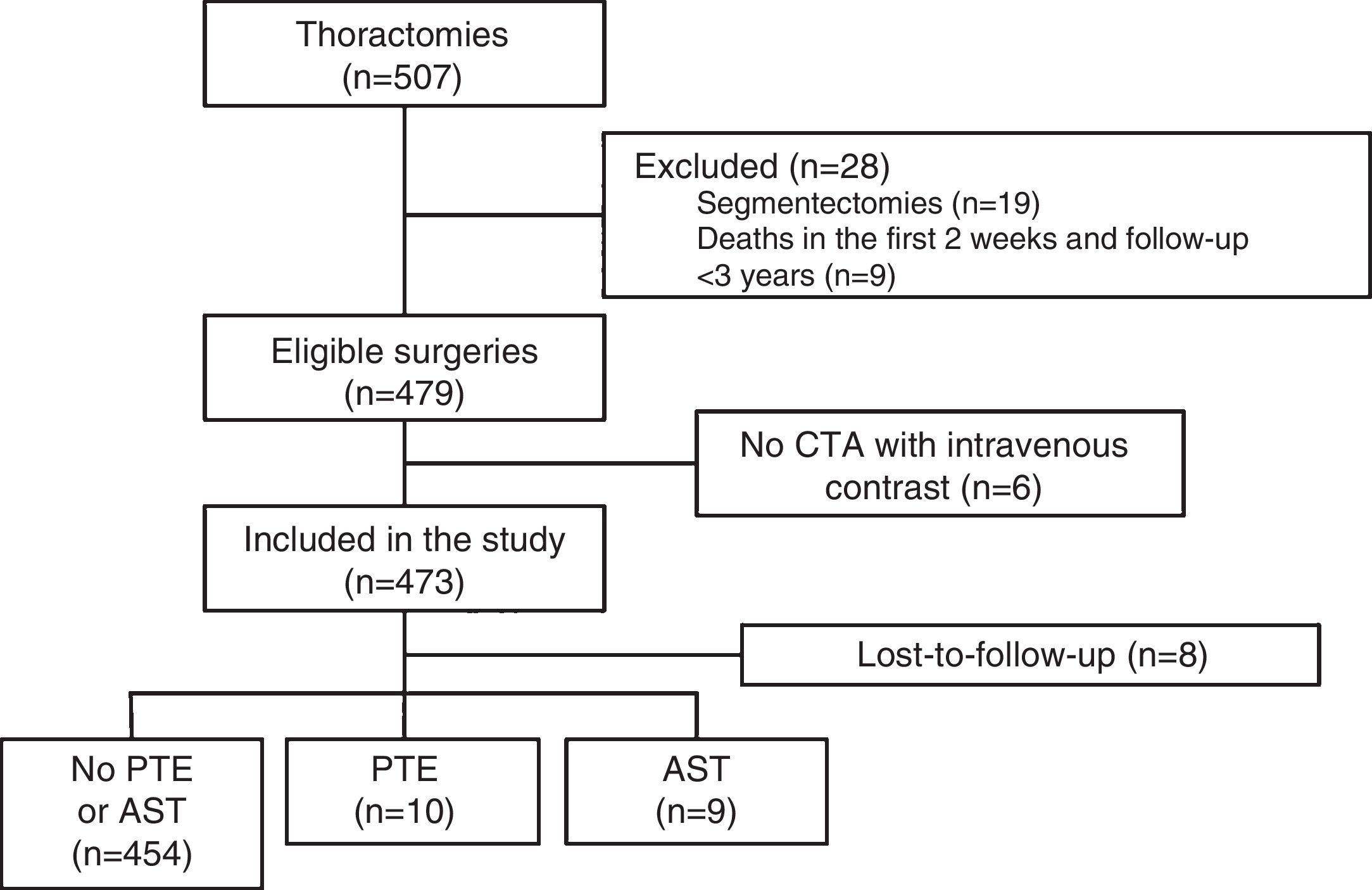
Study Setting and PopulationThe Hospital Universitario 12 de Octubre is a tertiary-level university teaching hospital with a reference population of approximately 500000 inhabitants. Patients were considered eligible if they had undergone lung resection surgery between January 2006 and June 2012, and included if they met the following criteria: (a) a clinical diagnosis of lung cancer before surgery; (b) treated with open thoracotomy and anatomical resection (lobectomy or pneumonectomy); (c) follow-up for at least 3 years; and (d) availability of computed tomography angiogram (CTA) with intravenous contrast medium during post-surgical follow-up. Patients who died in the 2 weeks following surgery and patients with sublobar resections were excluded. Follow-up concluded on May 25, 2015, and data collection was performed between June and August 2015.
The cohort was generated from records of thoracic surgery interventions performed during the study period, while follow-up data and other clinical and survival variables were obtained from a review of all clinical reports available in the paper and digital files of our hospital and other centers in the Community of Madrid, using the HORUS program, including emergency room visits, hospital admissions, and outpatient clinic consultations. Radiological variables were collected from an examination of the imaging studies and reports of all procedures performed in these patients retrieved from the digital database of our hospital. In accordance with SEPAR guidelines on the prophylaxis of venous thromboembolic disease (VTED),13 all surgeries were categorized as high risk, with the use of physical measures (compression methods) during admission and administration of low molecular weight heparin 2–6h before surgery. With regard to clinical follow-up, patients were seen at 2 weeks, 3 months, 6 months and 12 months after surgery. Subsequent visits were annual. Follow-up imaging studies were performed using CTA with intravenous contrast in the visits conducted 3 and 12 months and then every year.
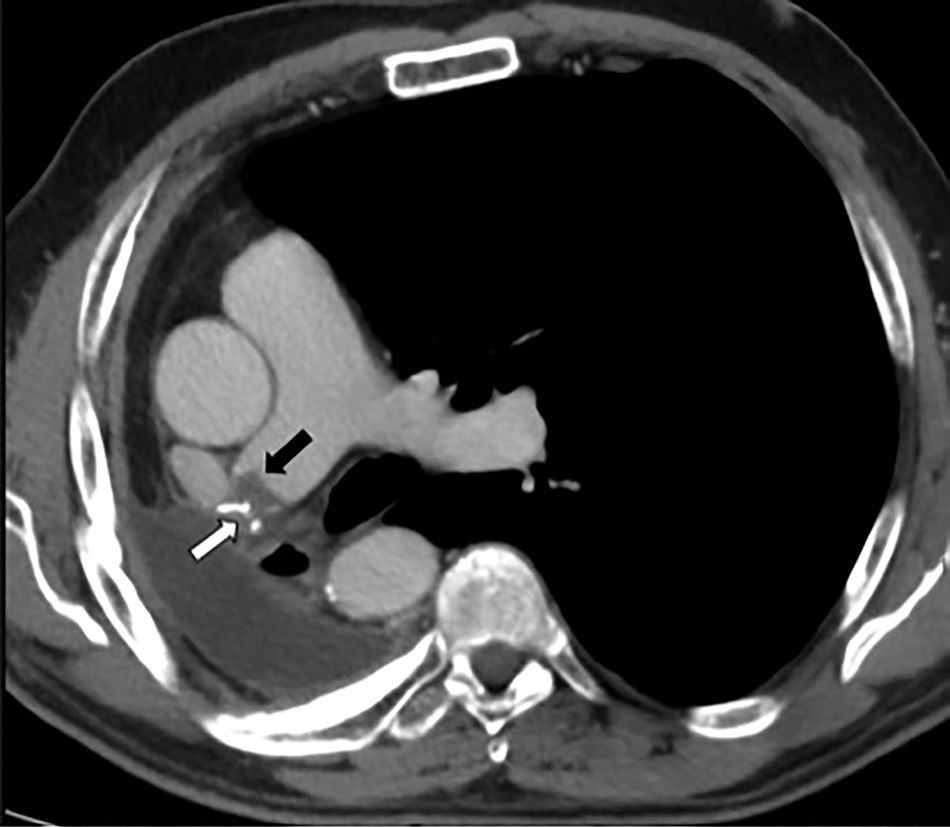
Variables AnalyzedOutcome variables: The primary variable was clot on the arterial stump observed on radiological studies, defined as the formation of a clot located and adhered to the arterial suture in lung resection surgeries, manifesting as a visible filling defect in the artery lumen. The outcome variable for the survival analysis was all-cause death.
Other clinical, surgical, oncological and radiological variables were collected.
Clinical variables: VTED, whether deep vein thrombosis (DVT) or PTE, the latter defined as a clot that has broken free (embolism) from another part of the venous system and lodged in the pulmonary arteries14; arterial hypertension (systolic/diastolic >140/90mmHg or antihypertensive treatment); diabetes mellitus (fasting blood sugar >125mg/dl on 2 or more determinations or glucose-lowering treatment); atrial fibrillation (diagnosed by electrocardiogram and/or antiarrhythmic treatment); obesity (body mass index ≥30kg/m2) and chronic airflow obstruction (maximum forced expiratory volume in 1 second [FEV1]<80%) (all variables: yes/no).
Surgical and radiological variables: type of intervention (right or left upper or lower lobectomy, middle lobectomy, bilobectomy and right or left pneumonectomy); if arterial reconstruction was required; number of CTAs with intravenous contrast; post-surgical pulmonary hypertension diagnosed by transthoracic echocardiography.
Oncological variables: histological strain and oncological staging according to international classifications15,16 with pathology report of surgical specimen; tumor relapse and death.
PTE variables: the following data were collected from patients with documented AST: antiplatelet treatment; radiological resolution and hypercoagulability disorder (yes/no); duration of antiplatelet treatment in days, and time between surgery and appearance and resolution of clot in months, if applicable.
Clinical variables before surgery were collected from the surgery discharge report, and other variables from the databases listed in the section “Study setting and population”. CTAs were reviewed by 2 radiologists with experience in cardiothoracic radiology and diagnosis of PTE (EPG and TYRO), and staging was confirmed consensually by 2 independent investigators (DLP and JSC).
StatisticsThe statistical analysis was performed using SPSS version 21.0 (SPSS Inc., Chicago, IL). Patients were divided into 3 groups: “no PTE or AST”, “PTE” and “AST”. Qualitative variables are presented as absolute numbers and percentages, while quantitative variables are given as means±standard deviations or medians with interquartile ranges, as applicable. Normal distribution of the variables was evaluated using the Kolgomorov–Smirnov test. The difference between the dichotomous characteristics of the study groups is presented as odds ratio (OR) calculated by logistic correlation and by the Student's t test for independent variables or the Mann–Whitney U test for quantitative variables, depending on their distribution. A P-value <.05 was considered statistically significant.
Secondary analyses included a survival analysis of the 3 study groups using the Kaplan–Meier test and compared using the log-rank tests after surgery, taking into account surgical and oncological clinical variables, for the analysis of patients with AST divided into 2 subgroups: “within 12 months” and “after 12 months” post-surgery.
Patients in whom the principal variable could not be established at the end of the study, i.e., AST, were considered “lost-to-follow-up”. However, they were not excluded from the final analysis, and the information entered in the computer system during their last contact with healthcare personnel was included in statistical calculations. Finally, no sensitivity analysis was planned, as few patients were expected to present the primary study variable.
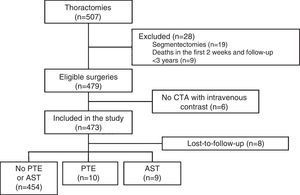
ResultsDuring the study, 507 thoracotomies were performed, of which 19 cases (4%) were excluded because they were segmentectomies; 9 patients (1.8%) were excluded because they died in the first 2 weeks after the intervention; and 6 (1.2%) were excluded because no CTA with intravenous contrast medium was performed during follow-up. Thus, a total of 473 surgeries were included, with final diagnoses of lung cancer in 471 cases (99.5%), cutaneous T cell lymphoma with pulmonary involvement in 1 patient and fungal infection in another. Resection type was lobectomy in 393 (83%), pneumonectomy in 61 (13%), and simultaneous bilobar resections in 19 (4%). The most common intervention type was right upper lobectomy (147 patients, 31%) and the most common histological strain was squamous cell carcinoma (213 patients, 45%). A total of 388 patients were men (82%), and mean age was 65.3±9.5 years. The patient selection flow chart is shown in Fig. 1 and other baseline characteristics in Table 1.
Pre-surgery Baseline Characteristics.
| Total Population (n=473) | Without AST (n=464) | AST (n=9) | OR (95% CI) | P | |
|---|---|---|---|---|---|
| Men | 388 (82) | 379 (82) | 9 (100) | 1.79 (0.22–14.56) | .58 |
| Age (years) | 65.3 (9.5) | 65.3 (9.5) | 66 (6.8) | 1.01 (0.94–1.09) | .71 |
| Previous VTED | 24 (5) | 23 (5) | 1 (11) | 2.17 (0.26–18.13) | .47 |
| Arterial hypertension | 208 (44) | 203 (44) | 5 (56) | 1.67 (0.44–6.31) | .45 |
| Diabetes mellitus | 85 (18) | 83 (18) | 2 (22) | 1.36 (0.28–6.70) | .70 |
| FEV1<80% | 203 (43) | 197 (42) | 6 (67) | 2.78 (0.69–11.28) | .15 |
Results are presented as absolute numbers (%) or mean (standard deviation).
AST: arterial stump thrombosis; VTED: venous thromboembolic disease; FEV1: forced expiratory volume in 1 second.
No significant differences were found among any comparisons performed.
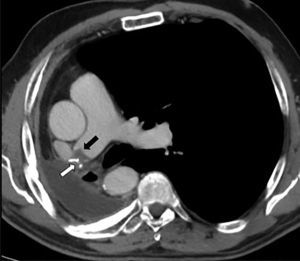
PET was diagnosed in 10 cases (2.1%) and AST in 9 (1.9%) (Fig. 2), resulting in an incidence of 1.9 cases of suture thrombosis per 100 patients operated. Table 2 shows surgical, radiological and oncological data by patient groups. Median follow-up after surgery was 49.4 months (interquartile range 34.8–70.1 months), and 147 deaths were documented. Table 3 describes causes for death by patient groups.
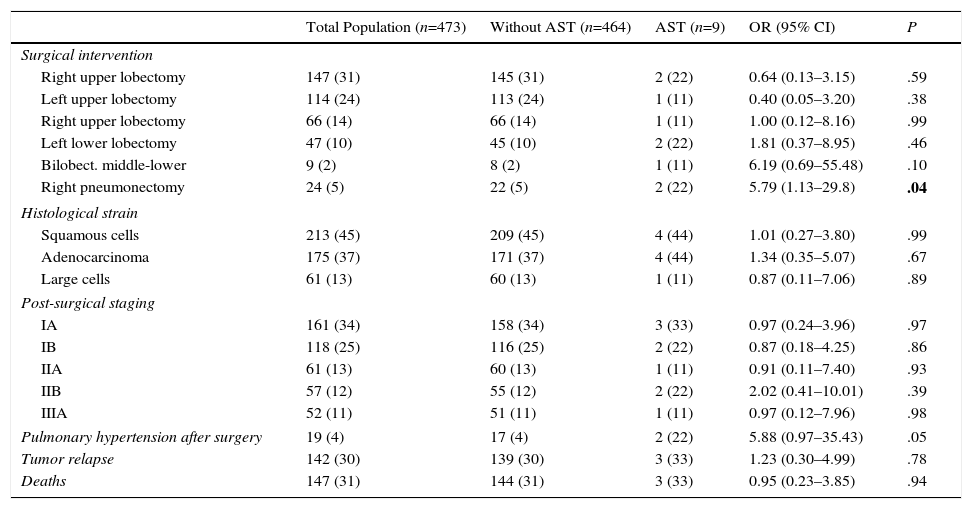
Surgical and Oncological Characteristics and Deaths in the 2 Groups, Classified According to Presentation of Arterial Stump Thrombosis.
| Total Population (n=473) | Without AST (n=464) | AST (n=9) | OR (95% CI) | P | |
|---|---|---|---|---|---|
| Surgical intervention | |||||
| Right upper lobectomy | 147 (31) | 145 (31) | 2 (22) | 0.64 (0.13–3.15) | .59 |
| Left upper lobectomy | 114 (24) | 113 (24) | 1 (11) | 0.40 (0.05–3.20) | .38 |
| Right upper lobectomy | 66 (14) | 66 (14) | 1 (11) | 1.00 (0.12–8.16) | .99 |
| Left lower lobectomy | 47 (10) | 45 (10) | 2 (22) | 1.81 (0.37–8.95) | .46 |
| Bilobect. middle-lower | 9 (2) | 8 (2) | 1 (11) | 6.19 (0.69–55.48) | .10 |
| Right pneumonectomy | 24 (5) | 22 (5) | 2 (22) | 5.79 (1.13–29.8) | .04 |
| Histological strain | |||||
| Squamous cells | 213 (45) | 209 (45) | 4 (44) | 1.01 (0.27–3.80) | .99 |
| Adenocarcinoma | 175 (37) | 171 (37) | 4 (44) | 1.34 (0.35–5.07) | .67 |
| Large cells | 61 (13) | 60 (13) | 1 (11) | 0.87 (0.11–7.06) | .89 |
| Post-surgical staging | |||||
| IA | 161 (34) | 158 (34) | 3 (33) | 0.97 (0.24–3.96) | .97 |
| IB | 118 (25) | 116 (25) | 2 (22) | 0.87 (0.18–4.25) | .86 |
| IIA | 61 (13) | 60 (13) | 1 (11) | 0.91 (0.11–7.40) | .93 |
| IIB | 57 (12) | 55 (12) | 2 (22) | 2.02 (0.41–10.01) | .39 |
| IIIA | 52 (11) | 51 (11) | 1 (11) | 0.97 (0.12–7.96) | .98 |
| Pulmonary hypertension after surgery | 19 (4) | 17 (4) | 2 (22) | 5.88 (0.97–35.43) | .05 |
| Tumor relapse | 142 (30) | 139 (30) | 3 (33) | 1.23 (0.30–4.99) | .78 |
| Deaths | 147 (31) | 144 (31) | 3 (33) | 0.95 (0.23–3.85) | .94 |
Results are presented as absolute numbers (%) or mean (standard deviation).
AST: arterial stump thrombosis; Bilobect.: bilobectomy; OR: odds ratio.
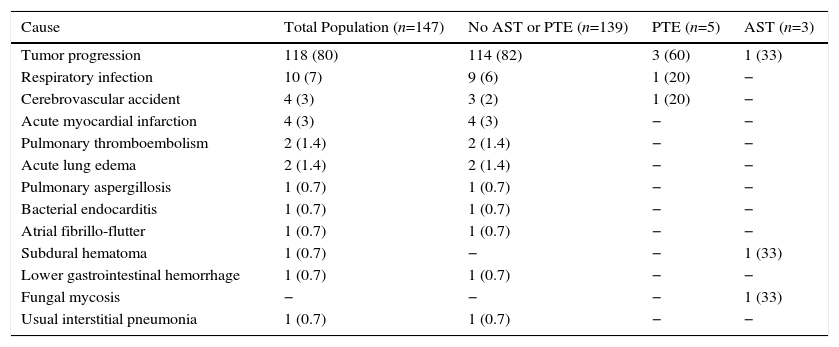
Causes of Death by Patient Groups.
| Cause | Total Population (n=147) | No AST or PTE (n=139) | PTE (n=5) | AST (n=3) |
|---|---|---|---|---|
| Tumor progression | 118 (80) | 114 (82) | 3 (60) | 1 (33) |
| Respiratory infection | 10 (7) | 9 (6) | 1 (20) | − |
| Cerebrovascular accident | 4 (3) | 3 (2) | 1 (20) | − |
| Acute myocardial infarction | 4 (3) | 4 (3) | − | − |
| Pulmonary thromboembolism | 2 (1.4) | 2 (1.4) | − | − |
| Acute lung edema | 2 (1.4) | 2 (1.4) | − | − |
| Pulmonary aspergillosis | 1 (0.7) | 1 (0.7) | − | − |
| Bacterial endocarditis | 1 (0.7) | 1 (0.7) | − | − |
| Atrial fibrillo-flutter | 1 (0.7) | 1 (0.7) | − | − |
| Subdural hematoma | 1 (0.7) | − | − | 1 (33) |
| Lower gastrointestinal hemorrhage | 1 (0.7) | 1 (0.7) | − | − |
| Fungal mycosis | − | − | − | 1 (33) |
| Usual interstitial pneumonia | 1 (0.7) | 1 (0.7) | − | − |
Results are presented as absolute numbers (%).
AST: arterial stump thrombosis; PTE: pulmonary thromboembolism.
After surgery, median time until AST was visualized was 11.3 months (interquartile range 2.7–42.2 months) with a range of 67.5 (1.4–69.0 months); of the 9 cases, 5 (56%) occurred within the 12 months following surgery. Seven (78%) of the AST patients began antiplatelet treatment. Three patients (33%) died, 2 of whom were receiving antiplatelets. Of these, 1 had a lymphoproliferative disorder, and 1 had an acute subdural hematoma while also receiving chemotherapy for tumor relapse. Median duration of antiplatelet treatment was 298 days (interquartile range 45–532 days). Filling defects were resolved, according to radiological findings, in 6 cases (67%), 2 of which were not receiving antiplatelets (22%). Media time to resolution was 12.9 months (interquartile range 3.8–20.3 months). Mean number of CTAs performed during follow-up of AST patients was 11.7±6.3 CTAs, statistically higher than the mean in patients without AST or PTE, who had 6.9±4.9 CTAs (P=0.02).
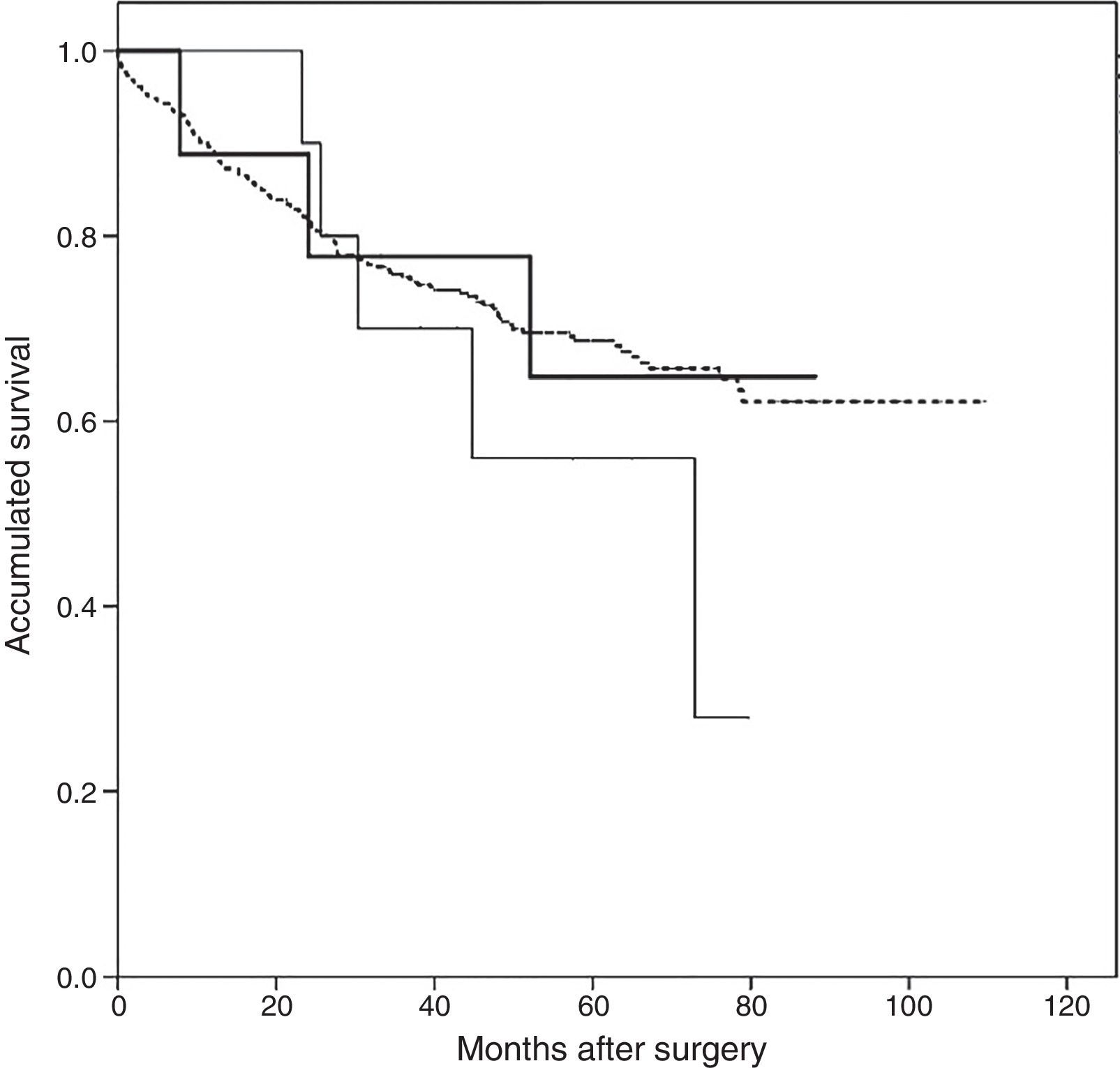
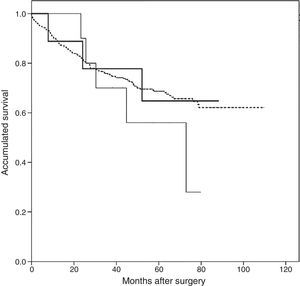
Follow-up after appearance of AST was 44 months (interquartile range 11.8–56.0 months). The Kaplan–Meier survival analysis revealed a greater accumulated survival in patients who did not present PTE or AST during follow-up, with a mean of 80.9 months (95% CI: 76.6–85.2 months), while patients with AST had a median survival of 67.4 months (95% CI: 47.4–87.4), and patients with PTE had the shortest survival at 56.9 months (95% CI: 42.1–71.8). However, none of the differences was statistically significant (log-rank 1.148, P=.56) (Fig. 3).
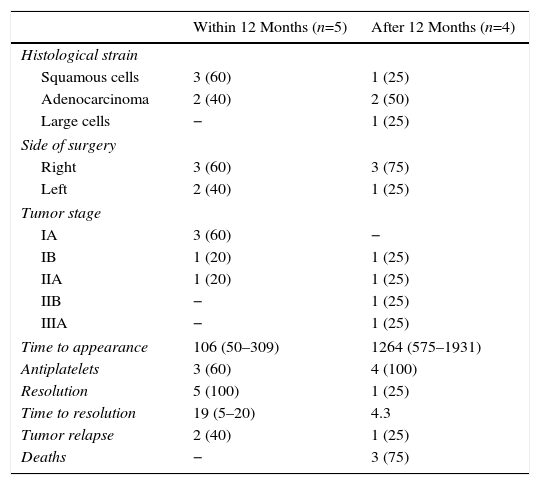
Finally, surgical, clinical and oncological characteristics of patients who developed AST, divided into subgroups of “within 12 months” following surgery and “after 12 months” following surgery, are presented in Table 4.
Oncological and Surgical Characteristics and Follow-up, Arterial Stump Thrombosis Occurring Before or After 12 Months Following the Surgical Intervention.
| Within 12 Months (n=5) | After 12 Months (n=4) | |
|---|---|---|
| Histological strain | ||
| Squamous cells | 3 (60) | 1 (25) |
| Adenocarcinoma | 2 (40) | 2 (50) |
| Large cells | − | 1 (25) |
| Side of surgery | ||
| Right | 3 (60) | 3 (75) |
| Left | 2 (40) | 1 (25) |
| Tumor stage | ||
| IA | 3 (60) | − |
| IB | 1 (20) | 1 (25) |
| IIA | 1 (20) | 1 (25) |
| IIB | − | 1 (25) |
| IIIA | − | 1 (25) |
| Time to appearance | 106 (50–309) | 1264 (575–1931) |
| Antiplatelets | 3 (60) | 4 (100) |
| Resolution | 5 (100) | 1 (25) |
| Time to resolution | 19 (5–20) | 4.3 |
| Tumor relapse | 2 (40) | 1 (25) |
| Deaths | − | 3 (75) |
Results are presented as absolute number (%) and median values (interquartile range), unless otherwise specified.
The most important result of our study was the determination of the epidemiological and clinical features of AST in lung resection surgery. AST was seen to be rare, with a tendency to occur in surgeries performed in the right side. It was not associated with pre-surgery risk factors, and was unrelated to histological strain, oncological staging or tumor relapse. Appearance over time was variable and it did not impact on patient survival.
With reference to prevalence, Kim et al.2 reported a total of 18 cases of AST in 147 pneumonectomies (12.2%), practically the same as that found by Kwek and Wittram3 who observed 11 clots in 89 patients (12.4%) also subjected to pneumonectomies. These authors focused their studies on the correlation between stump length and changes in blood flow dynamics, and included only pneumonectomies. Nevertheless, the prevalence of AST in this subgroup in our series is still low, with 2 cases among 61 patients (3.3%). The main reason for these different findings may be technical, since continuous suture was performed until the first semester of 2010, after which mechanical sutures with inert metal staples were used. A second reason may be compliance with a strict anticoagulation protocol associated with the surgery type and risk of VTED, proposed by the Spanish Society of Pulmonology and Thoracic Surgery.13 Our results, then, are closer to those of studies which have analyzed the prevalence of VTED in thoracic surgeries in general. In one series of almost 700 patients, a prevalence of 1.3% was determined, with no mention being made of AST,17 and in another Spanish series, a 0.18% prevalence was reported among 6,004 patients undergoing any type of chest surgery, with 7 cases of PTE and 4 of AST. It is worth noting that all cases of PTE had an underlying cancer diagnosis, and 6 of them had undergone major surgery, including pneumonectomies, lobectomies or atypical segmentectomies.18 With regard to the surgical technique, Işik et al.19 found that clot formation and embolization were more likely on transfixation sutures of left pneumonectomies, compared to continuous sutures.
The type of vessel sutured has been previously studied. While reports of embolic complications and infarctions in the case of AST are anecdotal, clots on venous sutures have been significantly associated with infarction, particularly in the brain. Recently, Yamamoto et al.20 presented a series of 6 patients who underwent left upper lobectomies complicated by cerebral infarction, of which 3 cases of clot on the corresponding vein were documented. Intraoperative ultrasonography has also shown that greater hemostasis occurs in the venous stump when the surgical procedure is left upper lobectomy.21 No infarction of vital organs was found in our series.
The association between the side of surgery and the appearance of AST has not been clearly determined. In contrast to the 2 studies mentioned above, our AST cases occurred more frequently in surgeries in the right side, as has also been reported in previous studies of pneumonectomies and AST [19 of the 29 cases detected (66%)].2,3 Moreover, in the small series and isolated clinical cases, right-side AST was observed in 6 of 7 cases of pneumonectomy presented (86%)4,5,7–9 and 1 case after right lower lobectomy.6 Following this line of argument, pneumonectomy as a major surgical procedure has been associated with greater activation of the coagulation cascade, and finally, with an increased chance of AST, compared to lobectomies.22
Our study revealed some interesting findings. AST occurring in the first 12 months after surgery was more likely to resolve, even in the 40% of cases which did not receive antiplatelet treatment. In contrast, those detected more than 1 year after surgery had a poor rate of resolution, and long-term antiplatelet treatments were prescribed in all cases. The number of cases is small, so no solid evidence is available on which to base the decision to administer antiplatelets and treatment duration. Adverse events associated with antiplatelet treatment included 1 case of acute subdural hematoma that developed while the patient was receiving active chemotherapy for tumor relapse, so it was impossible to rule out or confirm any association with anticoagulation. Logically, we also found a significantly greater number of CTAs conducted in patients with AST compared to patients without PTE or AST. However, no evidence could be found on the frequency or duration of radiological follow-up, despite the importance of collecting data on a radiological test that is far from risk-free and costly in terms of healthcare budgets.23
Our study has some limitations. Firstly, the loss of follow-up data commonly found in such retrospective studies limits the reliability of our results, although it should be pointed out that to date, none of the studies published on AST has been based on a prospective protocol. Secondly, the low number of cases detected reduces the power of the study and rules out a multivariate analysis for elucidating possible controllable risk factors. Previous series have similar limitations, and no multivariate analyses with more than 2 variables are available due to an insufficient rate of events.2,3 Both studies conclude that stump length is an associated factor. In our series, this measurement could not be determined in all cases as the CTA images obtained before 2008 were not routinely digitalized, and the technical characteristics of the tests were too varied. Thirdly, as mentioned above, this was not a prospective protocol, so patient follow-up was irregular in terms of CTA frequency, particularly during the first 4 years of the study. Moreover, CTAs were evaluated by different doctors, so failure to detect an AST cannot be ruled out.
Our study also has its strengths. This is a large series that included not only pneumonectomies but also other resection procedures, and, being the study with the longest follow-up published to date, it is also the first to present a survival analysis. The clinical relevance of this disease still remains unclear, so this series contributes to our knowledge of the frequency and course of this post-operative event.
To conclude, AST may be no more than an incidental finding, and it is true that its course is benign, but it is equally true that treatment and follow-up remain to be clarified. A larger case series would be desirable to help determine the identification, follow-up and therapeutic approach to this entity.
AuthorshipDaniel López-Padilla participated in data collection and is responsible for the integrity and accuracy of the analysis. He also contributed to the study design, interpretation, analysis and drafting of the final document. Esteban Peghini Gavilanes and Teresa Yolanda Revilla Ostolaza reviewed all the imaging studies to detect arterial stump thrombosis and contributed to the drafting of the final document. María Dolores Trujillo and Iván Martínez Serna retrieved all surgical interventions performed during the study period and contributed to the drafting of the final document. Nuria Arenas Valls, Walther Girón Matute, Roberto Larrosa Barrero, Adriana Manrique Multiozábal, Marta Pérez Gallán and Annette Zevallos collected data and contributed to the interpretation and drafting of the final document. Javier Sayas Catalán contributed substantially to the study design, interpretation, analysis and drafting of the final document.
Conflict of InterestsThe authors state that they have no conflict of interests.
Our thanks to Drs Pablo Gámez and Roberto del Pozo for their valuable methodological advice.
Please cite this article as: López-Padilla D, Gavilanes EP, Ostolaza TYR, Trujillo MD, Serna IM, Valls NA, et al. Trombosis en el muñón arterial de cirugías de resección pulmonar: análisis de su presentación clínica, tratamiento y evolución. Arch Bronconeumol. 2016;52:512–518.