We report the case of a 66-year-old woman with a 2-month history of dyspnea on exertion and self-limiting hemoptysis. She did not present cough, expectoration, or fever. She was a non-smoker with no other toxic habits, and did not report any history of environmental exposure to toxic substances. She had no clinical history of interest and was not receiving any previous treatment. Physical examination, including pulmonary auscultation, was normal. Her oxygen saturation, measured with pulse oximetry, was 95% breathing room air.
Blood tests showed hemoglobin 11.2mg/dl, C-reactive protein 168mg/dl, and fibrinogen 714mg/dl, with no other changes in hematological, biochemical, immunological or urinary tests. Lung function tests showed a restrictive pattern with reduced diffusion: FEV1 1.55 l (71% predicted) FVC 1.61 l (62%); FEV1/FVC 0.96; total lung capacity 3.64 l (73%); functional residual capacity 2.12 l (79%); lung diffusion capacity for carbon monoxide 3.8mmol/min/kPa (52%) and diffusion of carbon monoxide/alveolar volume 1.38mmol/min/kPa/l (92%).
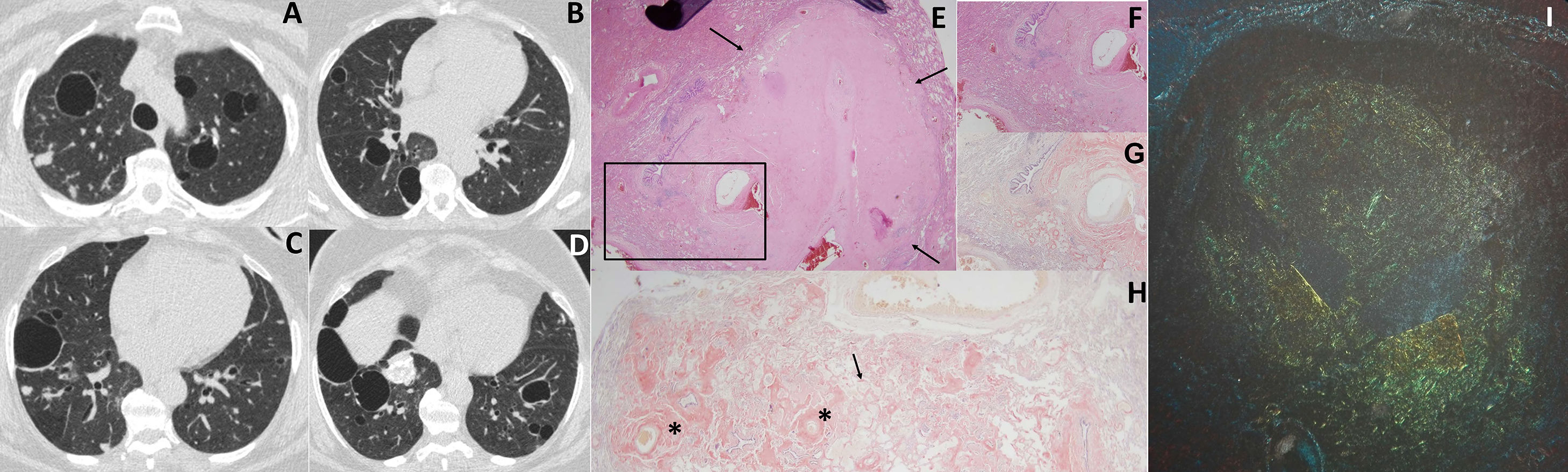
Given the findings of the chest X-ray, a chest computed tomography (CT) was performed, showing the presence of bilateral thin-walled air cysts and pulmonary nodules measuring 5–15mm in diameter, some of which were calcified (Fig. 1). No mediastinal lymphadenopathies were visualized. Positron emission tomography-CT showed no 18FDG uptake, reflecting lesions with a low level of metabolism, not suggestive of malignancy.
Chest CT with lung window (A–D) showing bilateral air cysts and nodules, some of which are calcified. Histological findings of lung biopsy (images E-I): (E) hematoxylin-eosin staining, 2×, showing pseudonodular areas (arrows) with tissue containing amorphous material; (F) enlarged image of sample (4×) showing amorphous eosinophilic tissue in the alveolar wall, near the blood vessel walls; (G) same sample with Congo red staining, 4×; (H) sample 2× stained with Congo red, showing amyloid protein in the blood vessel walls (asterisks) and in the alveolar wall (arrow); (I) apple-green birefringence under polarized light after Congo red staining.
A bronchoscopy was performed in which no endobronchial lesions were observed. Bronchoalveolar lavage (BAL) showed abundant histiocytes with CD68 expression. Microbiological cultures and cytology of the BAL were negative.
In view of these results, the patient was scheduled for a thoracoscopic lung biopsy of the right upper lobe. The histology study found pseudonodular areas with amorphous, acellular eosinophilic material that stained red with Congo red, and produced green birefringence under polarized light. The patient was diagnosed with pulmonary amyloidosis, negative for AA amyloid, suggestive of primary pulmonary amyloidosis (Fig. 1).
The patient was examined to identify possible hematological or collagen disease, but no associated disease was found. To date, the patient has not required treatment, and remains stable after 9 months of follow-up.
Amyloidosis comprises a group of diseases characterized by the abnormal deposition of fibrillary proteins in the extracellular matrix of several organs.1 They differ in the type of amyloid precursor protein; primary amyloidosis (AL) is the most common form (75% of cases), followed by secondary amyloidosis (AA) associated with other diseases, and familial or hereditary amyloidosis. The second form most commonly affects the lungs.2
There are several types of pulmonary amyloidosis, according to the site of deposition of the amyloid material. Tracheobronchial amyloidosis is the most common form, characterized by the infiltration of amyloid into the airway causing local or diffuse obstruction. Less common are the diffuse alveolar-septal form, characterized by amyloid deposits in the pulmonary interstitium, and the nodular form, in which amyloid creates pulmonary nodules called amyloidomas. Other less common forms of thoracic amyloidosis are adenopathic, laryngeal, or diaphragmatic involvement.2,3
The presentation of pulmonary amyloidosis as parenchymal air cysts associated with nodules is a rare mixed entity associated with AL amyloidosis, and tends to occur in women in the sixth decade of life with underlying collagen disease.4
The clinical picture of pulmonary amyloidosis is non-specific. When symptoms are present, the most typical is dyspnea on exercise, followed by cough, expectoration, hemoptysis, and self-reported wheezing.5 In a series of patients with pulmonary amyloidosis with cysts, only 29% of subjects were asymptomatic at diagnosis. Up to 72% of patients had dyspnea, and 14% had hemoptysis.6
Lung function in these patients is variable. Lung function is mostly normal (42%), followed by a pattern of restriction (32%), while only 11% of patients have reduced carbon monoxide diffusion.6 Localized pulmonary cystic amyloidosis is occasionally associated with vascular connective tissue diseases, such as Sjögren's syndrome, or more rarely, MALT lymphoma.7
Chest X-ray is normal in half of patients with cystic pulmonary amyloidosis. Chest CT shows multiple fine-walled air cysts, measuring 0.5–2cm in diameter, generally bilateral and symmetrical, predominantly in the lung bases.8 They are often accompanied by solid or subsolid pulmonary nodules that are frequently calcified.8–11 The radiological differential diagnosis should include lymphoid interstitial pneumonia, histiocytosis X, light-chain deposition disease, and Birt-Hogg Dubé syndrome.12
The diagnosis of pulmonary amyloidosis requires histological examination of lung biopsy specimens.13 Amyloid appears in the samples as a fibrous, amorphous, eosinophilic material with hematoxylin-eosin staining, that stains red with Congo red and typically shows green birefringence under polarized light microscopy. Primary and secondary pulmonary amyloidosis can be distinguished, since AA amyloid loses the birefringence associated with Congo red staining after oxidation in a potassium permanganate solution, while AL amyloid retains it. Nowadays, however, immunohistochemical techniques are used to typify amyloidosis using antibodies against immunoglobulin kappa and lambda light chains (AL amyloidosis), A protein (AA amyloidosis), transthyretin, and beta2-immunoglobulin.13
Treatment depends on the severity of the disease, and ranges from clinical observation to different therapeutic bronchoscopy procedures, including bronchial stent placement or ablative therapy with yttrium aluminum garnet (YAG) laser, argon plasma coagulation (APC), high-frequency electrocautery, and CO2 cryotherapy. Radiation therapy and surgical resection have also been used.14,15
In conclusion, this clinical case illustrates an example of primary pulmonary amyloidosis presenting as cystic lung disease associated with pulmonary nodules. The findings on imaging tests and the symptoms of these patients are non-specific, underlining the importance of including amyloidosis in the differential diagnosis of self-limiting hemoptysis, along with lung infections, bronchiectasis, vasculitis, and tumors.
Please cite this article as: García-Sánchez A, Villasante C, Esteban-Rodriguez I, F FG-R. Amiloidosis como causa de enfermedad pulmonar quística asociada a nódulos pulmonares. Arch Bronconeumol. 2018;54:481–482.