Comorbidities are thought to have prognostic impact on outcomes of patients submitted to noninvasive ventilation (NIV). Our goal was to determine if age-adjusted Charlson comorbidity index (ACCI) could predict outcomes in patients undergoing NIV due to acute respiratory failure.
MethodsPatients in respiratory failure submitted to NIV were prospective evaluated comparing patient's characteristics and outcomes according to ACCI≤median vs. ACCI>median. Each comorbidity composing the index was tested as predictor of NIV failure and readmission/mortality risk at 30 and 90 days, using logistic regression analysis. NIV failure was defined as need for invasive mechanical ventilation and/or death.
Results177 patients were enrolled. Median ACCI score was 5 points. Comparing patients with ACCI>5 with ACCI≤5, the former group was older but APACHE II was similar. Time to first NIV disconnection was inferior for ACCI>5 patients (OR 0.46, 95% CI 0.23–0.89, p=0.021), after gender and age adjustment. No differences were found in length of stay, time on NIV, NIV complications or failure, and 30 and 90-day hospital readmission or death, before and after adjustment. None of the single comorbidities was predictive of NIV failure and readmission risk, when adjusted to sex and age.
ConclusionACCI is not a good predictor for short and medium-term outcomes in patients submitted to NIV.
Las comorbilidades parecen tener un impacto en el pronóstico de los resultados de pacientes sometidos a ventilación no invasiva (VNI). Nuestro objetivo fue determinar si el ajuste por edad del índice de comorbilidad de Charlson (ICC) podía predecir los resultados de aquellos pacientes sometidos a VNI por insuficiencia respiratoria aguda.
MétodosSe evaluaron de forma prospectiva pacientes con insuficiencia respiratoria sometidos a VNI, comparando las características de los pacientes y sus resultados valorados como ICC<mediana vs. ICC>mediana. Mediante un análisis de regresión logística, cada comorbilidad incluida en el índice se evaluó como predictor de fracaso de VNI y de riesgo de reingreso/mortalidad a los 30 y 90 días. Se definió fracaso de VNI como la necesidad de ventilación mecánica asistida y/o muerte.
ResultadosSe incluyeron 177 pacientes. La mediana del valor del ICC fue de 5 puntos. En la comparación de pacientes con ICC>5 frente a pacientes con ICC≤5, el primer grupo resultó ser el de mayor edad, aunque la clasificación por APACHE II fue similar. El tiempo hasta la primera desconexión de VNI fue inferior en pacientes con ICC>5 (OR: 0,46; IC 95%: 0,23-0,89; p=0,021), tras el ajuste por género y edad. No se encontraron diferencias en la duración de la hospitalización, el tiempo con VNI, complicaciones o fracaso de la VNI y el reingreso hospitalario o la muerte a los 30 y 90 días, antes y después del ajuste. Ninguna de las comorbilidades analizadas era predictora de fracaso de la VNI o del riesgo de reingreso cuando se ajustaron por sexo edad.
ConclusiónEl ICC no es un buen predictor de los resultados a corto y medio plazo de pacientes sometidos a VNI.
Non-invasive ventilation (NIV) has changed the outcomes and prognosis of patients in respiratory failure and is now considered a first line treatment for acute hypercapnic respiratory failure. There is strong evidence that the addition of NIV to standard care improves outcomes in patients with chronic obstructive pulmonary disease exacerbation (COPDe) and acute cardiogenic pulmonary edema (ACPE)1,2 and the technique has been used to support successfully patients with acute respiratory failure from other aetiologies.3 The success rate can reach 85%2–5 and is shown to reduce the need for invasive mechanical ventilation, hospital length of stay and in-hospital mortality.6,7 Treatment with bilevel-positive airway pressure results in faster improvement in the PaO2/FiO2 ratio, subjective dyspnea score, respiratory and heart rates, and metabolic disturbances compared with oxygen alone.3,6,8,9 Recently, high-flow oxygen was described as an alternative to NIV in acute hipoxemic respiratory failure, but its use is not widespread.10 Due to its proven benefits, the use of NIV to treat COPDe increased more than 400% between 1998 and 2008.4
However, rates of failure, usually defined as need for invasive mechanical ventilation and death, are still significant.11,12 Identification of patients in whom NIV would be unsuccessful, either for causing delay in intubation or inducing unnecessary distress in poor prognostic cases, is determinant. Recent studies have evaluated the impact of prognostic factors in NIV-submitted patients such as severity of acute respiratory failure11,13 the response in the first hours of treatment,6,14 Acute Physiology and Chronic Health Evaluation (APACHE) II score13–15 and biomarkers.14,15
Comorbidities prevalence increases markedly with aging and is thought to have a prognostic impact. Several scores have been designed to evaluate their impact. Charlson comorbidity index (CCI) is a predictive score of 10-year mortality rate composed by specific comorbidities.16 As age is a known risk factor for medical outcome, in 1994 was published age-adjusted Charlson comorbidity index (ACCI), a score designed to evaluate peri-operative risk complications and long-term prognosis.17 Several studies have reported the accuracy of CCI to determine risk of complications and mortality.18–20
Whether the presence of chronic comorbidities could influence NIV success and short and medium-term related mortality was only partially investigated.21,22 Our aim is to prospectively evaluate the ACCI as a predictor of outcomes in patients submitted to NIV and determine if this tool could help to decide which patients could benefit more from the therapy.
Patients and methodsThis is a prospective observational study of a cohort of patients admitted to an Intermediate Care Unit of a tertiary institution, submitted to NIV for acute medical illness between September 2014 and December 2016. We included patients with 18 or more years and accepted all causes of respiratory failure. Patients submitted to invasive mechanical ventilation in which NIV was used for weaning were excluded.
Data collectionData was collected from patients and medical records. Demographic data, comorbid illnesses, physical examination data, blood tests at start on NIV, etiology of respiratory failure, ventilatory parameters, time to first disconnection, days on NIV, and length of stay were record. Order to “do not intubate” was collected with the patient's medical team, without any investigators intervention on the decision.
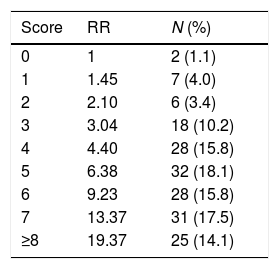
Age-adjusted Charlson comorbidity indexThe overall comorbid status of each patient was quantified using the ACCI,17 which is a summation score, age adjusted, based on 19 medical conditions with varying points assigned. A score of 1 is given to each of the following conditions: myocardial infarction, cardiac failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, mild liver disease, and diabetes without organ damage; a score of 2 for: hemiplegia, moderate to severe renal disease, any tumor within last 5 years, lymphoma, leukemia and diabetes with organ damage; a score of 3 for: moderate to severe liver disease; and a score of 6 for metastatic solid tumor and acquired immunodeficiency syndrome. Each decade of age ≥50 years is equivalent to a 1-point increase in the score. A value of 0 indicates no comorbidity, while higher values represent an increasing burden of comorbid illnesses. The relative risk of death for each decade of age is 1.42 and for each increasing comorbidity rank was 1.46, with a combined risk of 1.45 for each point in the rank. Relative risk (RR) of mortality increases with ACCI score: 0 point=RR 1, 1 point=RR 1.45, 2 points=RR 2.10, 3 points=RR 3.04, 4 points=RR 4.40, 5 points=RR 6.38, 6 points=RR 9.23, 7 points=RR 13.37, ≥8 points=RR 19.37. As we expect to find high comorbidity burden in our population, we used the median AACI as the cut-off for comparing patients with “low” risk (ACCI≤median) and “high” risk (ACCI>median) profiles.
Outcomes and study endpointsPrognosis was assessed comparing complications between groups during hospitalization (NIV intolerance, mask associated facial ulcers, aspiration, need for invasive mechanical ventilation, death and others) and after discharge (readmission/time to readmission and death/time to death at 30 days and 90 days). Time to first disconnection, NIV days, and length of stay (total and after NIV) were also accessed.
The primary end-point was all-cause mortality according to “low” or “high” risk ACCI group. Secondary end-points were NIV complications and hospital readmission according to “low” or “high” risk ACCI group.
Statistical analysisDescriptive statistics are expressed as mean with standard deviation or median with interquartile range (IQR) for continuous variables. Categorical data are presented as frequency. Demographic and laboratorial data were compared between the two groups (ACCI≤median vs. ACCI>median) on continuous variables using t-tests or Median test and on categorical variables using χ2 tests or Fisher exact test, as appropriate. Univariate and odds ratio (OR) were estimated with logistic regression for identifying the comorbidities associated with NIV outcome. The results were adjusted to gender and age. A p value less than 0.05 was considered statistically significant. Statistical analyses were carried out using IBM® SPSS® Statistical Software, version 24.0.
Ethical considerationsThe study complied with the Declaration of Helsinki, and the institutional ethics board approved the study protocol. All patients signed an informed consent.
ResultsDuring study period were admitted 404 patients to NIV at intermediate care unit. One hundred and seventy-seven patients signed consent. Fifty-four percent were male and median age was 76.0±19.5 years old (range, 34–98 years). Ninety percent of patients were admitted due to non-surgical acute illness, 5% due to urgent surgical disease and 5% due to elective surgical disease. The major diagnosis leading to institution of NIV was respiratory failure do to ACPE (54.2%), COPDe (23.2%) and other respiratory diseases (15.3%). One hundred and seventy-three patients were ventilated in a pressure support mode and the others in pressure controlled mode.
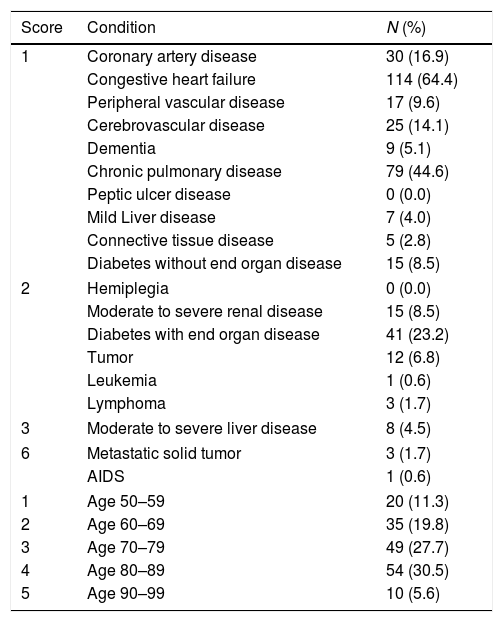
The mean ACCI score was 5.4±2.5 points and the median value was 5 points. Ninety-two percent of the patients had, at least, one comorbidity. Patients ACCI distribution and respective comorbid conditions are characterized in Tables 1 and 2, respectively.
Patients age-adjusted Charlson comorbidity index and comorbid conditions.
| Score | Condition | N (%) |
|---|---|---|
| 1 | Coronary artery disease | 30 (16.9) |
| Congestive heart failure | 114 (64.4) | |
| Peripheral vascular disease | 17 (9.6) | |
| Cerebrovascular disease | 25 (14.1) | |
| Dementia | 9 (5.1) | |
| Chronic pulmonary disease | 79 (44.6) | |
| Peptic ulcer disease | 0 (0.0) | |
| Mild Liver disease | 7 (4.0) | |
| Connective tissue disease | 5 (2.8) | |
| Diabetes without end organ disease | 15 (8.5) | |
| 2 | Hemiplegia | 0 (0.0) |
| Moderate to severe renal disease | 15 (8.5) | |
| Diabetes with end organ disease | 41 (23.2) | |
| Tumor | 12 (6.8) | |
| Leukemia | 1 (0.6) | |
| Lymphoma | 3 (1.7) | |
| 3 | Moderate to severe liver disease | 8 (4.5) |
| 6 | Metastatic solid tumor | 3 (1.7) |
| AIDS | 1 (0.6) | |
| 1 | Age 50–59 | 20 (11.3) |
| 2 | Age 60–69 | 35 (19.8) |
| 3 | Age 70–79 | 49 (27.7) |
| 4 | Age 80–89 | 54 (30.5) |
| 5 | Age 90–99 | 10 (5.6) |
AIDS: acquired immunodeficiency syndrome.
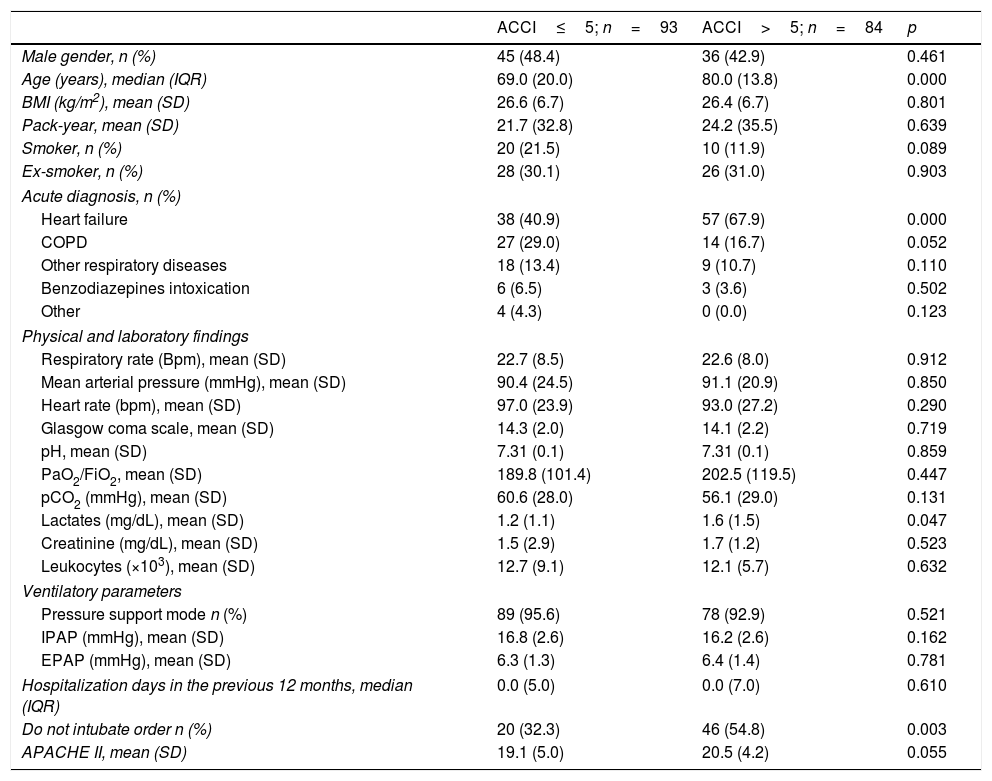
Table 3 compares patients with ACCI>5 and ACCI≤5. The acute diagnosis was similar between the groups except for acute heart failure which was significantly more frequent in ACCI>5 group. Physical examination, laboratory findings and ventilatory parameters were comparable. The frequency of hospital admissions the year before was 34.7% for ACCI≤5 and 39.3% for ACCI>5 group (p=0.556). APACHE II score was superior in the ACCI>5 group but the difference was not significant. ACCI>5 group had more therapeutic limitations, namely to invasive mechanical ventilation.
Baseline characteristics of patients submitted to NIV by age-ajusted Charlson Comorbidity Index group.
| ACCI≤5; n=93 | ACCI>5; n=84 | p | |
|---|---|---|---|
| Male gender, n (%) | 45 (48.4) | 36 (42.9) | 0.461 |
| Age (years), median (IQR) | 69.0 (20.0) | 80.0 (13.8) | 0.000 |
| BMI (kg/m2), mean (SD) | 26.6 (6.7) | 26.4 (6.7) | 0.801 |
| Pack-year, mean (SD) | 21.7 (32.8) | 24.2 (35.5) | 0.639 |
| Smoker, n (%) | 20 (21.5) | 10 (11.9) | 0.089 |
| Ex-smoker, n (%) | 28 (30.1) | 26 (31.0) | 0.903 |
| Acute diagnosis, n (%) | |||
| Heart failure | 38 (40.9) | 57 (67.9) | 0.000 |
| COPD | 27 (29.0) | 14 (16.7) | 0.052 |
| Other respiratory diseases | 18 (13.4) | 9 (10.7) | 0.110 |
| Benzodiazepines intoxication | 6 (6.5) | 3 (3.6) | 0.502 |
| Other | 4 (4.3) | 0 (0.0) | 0.123 |
| Physical and laboratory findings | |||
| Respiratory rate (Bpm), mean (SD) | 22.7 (8.5) | 22.6 (8.0) | 0.912 |
| Mean arterial pressure (mmHg), mean (SD) | 90.4 (24.5) | 91.1 (20.9) | 0.850 |
| Heart rate (bpm), mean (SD) | 97.0 (23.9) | 93.0 (27.2) | 0.290 |
| Glasgow coma scale, mean (SD) | 14.3 (2.0) | 14.1 (2.2) | 0.719 |
| pH, mean (SD) | 7.31 (0.1) | 7.31 (0.1) | 0.859 |
| PaO2/FiO2, mean (SD) | 189.8 (101.4) | 202.5 (119.5) | 0.447 |
| pCO2 (mmHg), mean (SD) | 60.6 (28.0) | 56.1 (29.0) | 0.131 |
| Lactates (mg/dL), mean (SD) | 1.2 (1.1) | 1.6 (1.5) | 0.047 |
| Creatinine (mg/dL), mean (SD) | 1.5 (2.9) | 1.7 (1.2) | 0.523 |
| Leukocytes (×103), mean (SD) | 12.7 (9.1) | 12.1 (5.7) | 0.632 |
| Ventilatory parameters | |||
| Pressure support mode n (%) | 89 (95.6) | 78 (92.9) | 0.521 |
| IPAP (mmHg), mean (SD) | 16.8 (2.6) | 16.2 (2.6) | 0.162 |
| EPAP (mmHg), mean (SD) | 6.3 (1.3) | 6.4 (1.4) | 0.781 |
| Hospitalization days in the previous 12 months, median (IQR) | 0.0 (5.0) | 0.0 (7.0) | 0.610 |
| Do not intubate order n (%) | 20 (32.3) | 46 (54.8) | 0.003 |
| APACHE II, mean (SD) | 19.1 (5.0) | 20.5 (4.2) | 0.055 |
ACCI: age-adjusted Charlson comorbidity index; BMI: body mass index; IPAP: inspiratory positive airway pressure; EPAP: expiratory positive airway pressure; NIV: noninvasive ventilation; IMV: invasive mechanical ventilation.
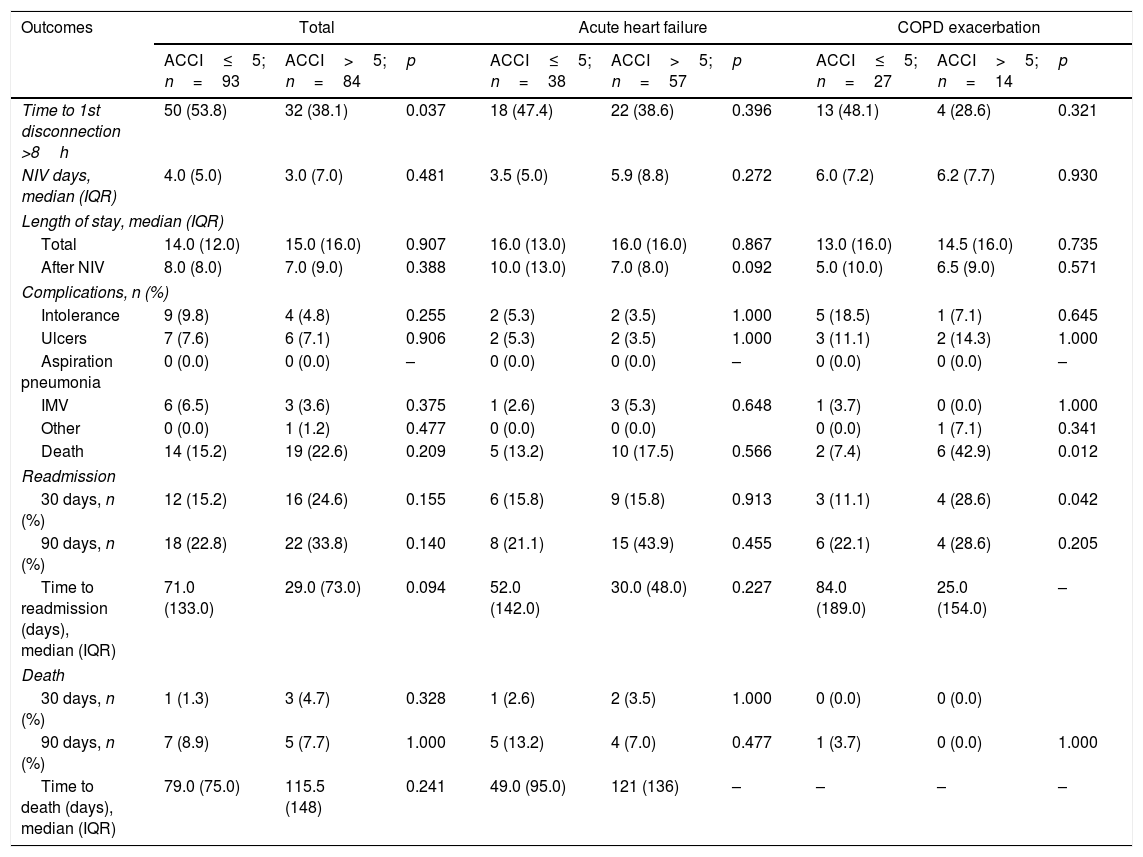
Outcomes (Table 4) were not different between groups except for time to first disconnection which was inferior in the higher risk group before (OR 0.53, 95% CI 0.29–0.96, p=0.038) and after (OR 0.46, 95% CI 0.23–0.89, p=0.021) adjusting to gender and age. None of the other outcomes was associated to ACCI group after adjustment.
Outcomes of patients submitted to NIV according to age-ajusted Charlson Comorbidity Index group.
| Outcomes | Total | Acute heart failure | COPD exacerbation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ACCI≤5; n=93 | ACCI>5; n=84 | p | ACCI≤5; n=38 | ACCI>5; n=57 | p | ACCI≤5; n=27 | ACCI>5; n=14 | p | |
| Time to 1st disconnection >8h | 50 (53.8) | 32 (38.1) | 0.037 | 18 (47.4) | 22 (38.6) | 0.396 | 13 (48.1) | 4 (28.6) | 0.321 |
| NIV days, median (IQR) | 4.0 (5.0) | 3.0 (7.0) | 0.481 | 3.5 (5.0) | 5.9 (8.8) | 0.272 | 6.0 (7.2) | 6.2 (7.7) | 0.930 |
| Length of stay, median (IQR) | |||||||||
| Total | 14.0 (12.0) | 15.0 (16.0) | 0.907 | 16.0 (13.0) | 16.0 (16.0) | 0.867 | 13.0 (16.0) | 14.5 (16.0) | 0.735 |
| After NIV | 8.0 (8.0) | 7.0 (9.0) | 0.388 | 10.0 (13.0) | 7.0 (8.0) | 0.092 | 5.0 (10.0) | 6.5 (9.0) | 0.571 |
| Complications, n (%) | |||||||||
| Intolerance | 9 (9.8) | 4 (4.8) | 0.255 | 2 (5.3) | 2 (3.5) | 1.000 | 5 (18.5) | 1 (7.1) | 0.645 |
| Ulcers | 7 (7.6) | 6 (7.1) | 0.906 | 2 (5.3) | 2 (3.5) | 1.000 | 3 (11.1) | 2 (14.3) | 1.000 |
| Aspiration pneumonia | 0 (0.0) | 0 (0.0) | – | 0 (0.0) | 0 (0.0) | – | 0 (0.0) | 0 (0.0) | – |
| IMV | 6 (6.5) | 3 (3.6) | 0.375 | 1 (2.6) | 3 (5.3) | 0.648 | 1 (3.7) | 0 (0.0) | 1.000 |
| Other | 0 (0.0) | 1 (1.2) | 0.477 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 0.341 | |
| Death | 14 (15.2) | 19 (22.6) | 0.209 | 5 (13.2) | 10 (17.5) | 0.566 | 2 (7.4) | 6 (42.9) | 0.012 |
| Readmission | |||||||||
| 30 days, n (%) | 12 (15.2) | 16 (24.6) | 0.155 | 6 (15.8) | 9 (15.8) | 0.913 | 3 (11.1) | 4 (28.6) | 0.042 |
| 90 days, n (%) | 18 (22.8) | 22 (33.8) | 0.140 | 8 (21.1) | 15 (43.9) | 0.455 | 6 (22.1) | 4 (28.6) | 0.205 |
| Time to readmission (days), median (IQR) | 71.0 (133.0) | 29.0 (73.0) | 0.094 | 52.0 (142.0) | 30.0 (48.0) | 0.227 | 84.0 (189.0) | 25.0 (154.0) | – |
| Death | |||||||||
| 30 days, n (%) | 1 (1.3) | 3 (4.7) | 0.328 | 1 (2.6) | 2 (3.5) | 1.000 | 0 (0.0) | 0 (0.0) | |
| 90 days, n (%) | 7 (8.9) | 5 (7.7) | 1.000 | 5 (13.2) | 4 (7.0) | 0.477 | 1 (3.7) | 0 (0.0) | 1.000 |
| Time to death (days), median (IQR) | 79.0 (75.0) | 115.5 (148) | 0.241 | 49.0 (95.0) | 121 (136) | – | – | – | – |
ACCI: age-adjusted Charlson comorbidity index; NIV: noninvasive ventilation; IMV: invasive mechanical ventilation.
Heart failure, COPD and other respiratory disease represented 93% of the diagnosis leading to NIV institution. Comparing outcomes of each subpopulation, the results were only different in patients with COPDe. Univariate analysis showed an association with hospital mortality for COPDe (OR 9.38, 95% CI 1.57–56.01, p=0.014) but not when adjusted to sex and age (OR 4.73, 95% CI 0.63–35.30, p=0.129). Readmission risk for COPDe patients was not different before (OR 3.17, 95% CI 0.60–16.7, p=0.174) and after (OR 1.74, 95% CI 0.27–11.10, p=0.560) adjusting to gender and age.
Table 5 describes outcomes according to each CCI comorbidity after adjusting to age and gender. None was associated with the need of invasive mechanical ventilation. Mortality after discharge was associated with mild liver disease (OR 14.40, 95% CI 1.08–192.90, p=0.044), when adjusted to age and sex, at 30 days, but the tendency disappears at 90 days. Only lymphoma was predictive of mortality at 90 days after discharge (OR 26.91, 95% CI 1.22–591.84, p=0.037) after adjustment. None of the comorbidities was predictive of readmission risk.
Outcomes of patients submitted to NIV by comorbidity (adjusted to gender and age).
| NIV failure | 30 days death | 90 days death | 30 days readmission | 90 days readmission | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Coronary artery disease | 1.00 (0.38–2.64) | 0.994 | 0.68 (0.20–2.28) | 0.530 | 0.78 (0.27–2.20) | 0.633 | ||||
| Congestive heart failure | 1.15 (0.50–2.64) | 0.741 | 1.35 (0.12–15.21) | 0.810 | 0.40 (0.10–1.54) | 0.182 | 1.84 (0.66–5.15) | 0.248 | 0.97 (0.42–2.24) | 0.951 |
| Peripheral vascular disease | 1.52 (0.49–4.73) | 0.471 | 0.90 (0.18–4.52) | 0.895 | 1.50 (0.41–5.46) | 0.542 | ||||
| Cerebrovascular disease | 0.42 (0.12–1.53) | 0.188 | 0.32 (0.04–2.72) | 0.297 | 0.95 (0.31–2.96) | 0.931 | 1.01 (0.37–2.78) | 0.989 | ||
| Dementia | 1.09 (0.21–5.79) | 0.915 | 1.14 (0.11–11.74) | 0.913 | 2.29 (0.44–12.06) | 0.328 | 2.69 (0.53–13.65) | 0.233 | ||
| Chronic pulmonary disease | 1.57 (0.73–3.37) | 0.246 | 1.71 (0.21–14.00) | 0.619 | 1.12 (0.32–4.23) | 0.812 | 2.35 (0.95–5.82) | 0.064 | 2.03 (0.92–4.48) | 0.079 |
| Mild Liver disease | 1.73 (0.31–9.64) | 0.531 | 14.38 (1.07–192.48) | 0.044 | 4.28 (0.40–46.47) | 0.232 | 1.25 (0.13–12.13) | 0.847 | 0.72 (0.08–6.81) | 0.774 |
| Moderate to severe liver disease | 1.21 (0.23–6.47) | 0.825 | 10.83 (0.82–142.20) | 0.070 | 2.52 (0.24–26.03) | 0.437 | 0.86 (0.09–8.05) | 0.891 | 0.54 (0.06–4.93) | 0.584 |
| Connective tissue disease | 3.85 (0.58–25.44) | 0.162 | 3.03 (0.25–36.31) | 0.381 | 1.59 (0.14–18.36) | 0.711 | ||||
| Diabetes without end organ disease | 1.01 (0.26–3.86) | 0.987 | 0.99 (0.11–8.63) | 0.989 | 0.76 (0.16–3.73) | 0.738 | 0.78 (0.20–3.02) | 0.715 | ||
| Diabetes with end organ disease | 0.46 (0.30–1.91) | 0.557 | 1.05 (0.11–10.54) | 0.967 | 1.01 (0.25–4.05) | 0.995 | 0.78 (0.28–2.14) | 0.623 | 1.74 (0.76–3.95) | 0.189 |
| Lymphoma | 1.89 (0.16–22.52) | 0.614 | 26.91 (1.22–591.84) | 0.037 | 6.35 (0.36–112.22) | 0.207 | 3.62 (0.21–62.02) | 0.374 | ||
| Moderate to severe renal disease | 1.03 (0.27–3.95) | 0.962 | 4.14 (0.38–45.70) | 0.246 | 1.11 (0.13–9.81) | 0.927 | 0.83 (0.17–4.07) | 0.815 | 0.83 (0.21–3.24) | 0.789 |
| Tumor | 1.02 (0.26–4.12) | 0.973 | 5.02 (0.37–67.61) | 0.224 | 0.83 (0.16–4.41) | 0.826 | 0.55 (0.11–2.83) | 0.472 | ||
| Metastatic solid tumor | 1.57 (0.14–18.19) | 0.720 | ||||||||
NIV: noninvasive ventilation.
General population is now living longer due to better health care. Nowadays, we see more and more hospitalized elders, with high comorbidity burden, that with appropriate therapy are discharged. NIV is an increasingly used therapy and, in those who are too frail to be submitted to IMV, represents an appropriate tool to manage acute respiratory failure. However, NIV has side effects and not all patients are able to tolerate it. Patients should be carefully selected to minimize hazards.
ACCI is not disease specific. In fact, it has been applied to study patients with cancer, renal disease, stroke and liver disease. ACCI can be used in acutely ill or chronic patients, both in clinical or research practice. It includes a variety of comorbidities, most of them very common, and to some of them attributes a different weight according to the severity of comorbidity. Moreover, this is the comorbidity score most commonly used in our hospital and, as it is widely used in other studies, allow result comparison. These make ACCI an interesting tool for its simplicity and reproductivity.
Our sample represents 43.8% of the patients admitted during study period. The median cohort age was 76 years, higher than in other NIV studies.23–25 Except for patients in ACPE, the diagnosis leading to NIV was similar between “high” and “low” risk groups. We did not find any difference in physical examination, laboratory findings and ventilatory parameters. In fact, APACHE II score was similar in both groups and the tendency to higher score values for ACCI>5 population is probably, at least, partially related to age, a parameter included in both APACHE II and ACCI. In fact, as the APACHE II values in the two groups are similar, and 64% of our sample has ≥70 years old, we suspect that excluding age from APACHE II score, patients with ACCI>5 probably would have lower APACHE score. This finding is extremely important as several studies have excluded age as a determinant prognostic factor. In fact, Liu et al. concluded that age, at the beginning of treatment, is a protective factor to NIV intolerance.23 Our intolerance rate was 7%, an analogous value to other works,23 validating these findings. Chloros et al. compared the outcomes of NIV by age, in patients with different acute diagnosis, and concluded that despite the need of more days of therapy in the elder, mortality was similar between groups.26 As neither comfort or outcomes seem to be compromised, age should not limit offering NIV.
Time to first NIV disconnection was significantly higher in the lower risk group but, attending to the higher prevalence of ACPE motivating NIV therapy, we think that the difference is due to the more rapid clinic response to medical therapy in these patients. We do not exclude that age could have had a role in this decision to minimize some hypothetical discomfort in the older patients.
Eighty-six to 98% of COPD patients have comorbidities.27–30 In patients with heart failure, especially in elder populations, the prevalence can reach 96%.31 Our sample has a high burden of comorbidities comparing to other published works.24,32–34 The reason to choose this high cut-off value related to the fact that our sample represents the population admitted to our intermediate care units. It would be unreal to use a smaller ACCI cut-off value to understand the outcomes of our patients.
It is now recognized that mortality in COPD and HF patients is often attributable to associated conditions. In COPD, cause-specific mortality at three years was linked to pulmonary causes in 35% of cases, cardiovascular causes in 27%, and cancer in 21%.35 Identification and stratification of patients according to severity of acute illness combined with chronic conditions would help clinicians to define prognosis and consequently management. However, we did not find impact of high or low comorbidity burden, using ACCI, on the outcomes. In fact, length of stay, number of days on NIV, number of complications (including death during hospitalization) and risk of readmission and mortality after discharge, were comparable.
In the subgroup analysis we found that COPD patients with higher ACCI had a significantly higher hospital mortality and early readmission risk. However, our sample was considerably small and the difference disappeared after adjusting to age and gender. We believe that we have a small COPDe population, contrary to literature reports, due to our respiratory clinic, which is easily accessible to patients, allowing sooner exacerbation identification, medical and ventilatory therapy adjustment and outpatient treatment.
Several studies utilized Charlson comorbidity index to describe comorbidity burden in COPD patients26,32,36 but some of them have questioned the validity of this index in the COPD-specific population. Almagro et al. showed that the Charlson index underestimated the burden of comorbidities in a Spanish population with COPD. In their work osteoporosis, anemia, arterial hypertension and alcoholism were identified as having a role in the disease and these are not included in ACCI.32 Pacilli et al. showed that the number of comorbidities was higher in NIV failure group when compared to those patients who succeeded.22 However, except for dementia, none of the Charlson index comorbidities was, by itself, associated with unfavorable outcomes. In fact, the authors concluded that Charlson index had a very weak predictive value of unsuccess in a multivariate model. Several new scores, incorporating comorbidities have been created to mitigate these limitations and include new dimensions such as psychiatric comorbidities.37,38
ACPE patients submitted to NIV in our study had the same outcomes independently of ACCI score. Nowadays, there is not sufficient data concerning the indication of NIV in elderly patients admitted for ACPE, considering their multiple comorbidities and performance status limitations. Calvo et al.39 proved NIV as a successful therapy even in elderly patients with multiple comorbidities. In this study, survivors and deceased patient's Charlson score was 3.8 and 3.9 points, respectively (p=0.971). Interestingly, 52% of this sample was at least partially dependent in basic daily activities and the chances of survival were inferior in the more functionally limited patients. In another study, 1-year cumulative survival and quality of life were inferior in patients with lower Barthel index scores.40 This supports that dependency status is a crucial prognostic parameter and should be included in future prognostic scores besides comorbidities.
CCI score was published 30 years ago. As in this time, survival has increased significantly, the adjustment proposed by the original manuscript could not be representative of the reality. This is the reason why we performed an age readjustment. Another limitation of ACCI is that the severity of some of the comorbidities is not defined. For example, we believe that HF is undoubtedly a prevalent and significant prognostic comorbidity. However, ACCI considers all symptomatic patients with response to medical therapy, pooling together NYHA class II to IV HF patients, which have a very different prognosis.
In our hospital, NIV in an acute setting is successfully performed in general wards, especially in populations that due to their comorbidities and severity of acute illness are not candidates to be admitted to more differentiated care units. In fact, according to our hospital ICD 9 codification results, 790 patients were submitted to NIV during patient's enrolment, stating that almost 50% of the technique is performed outside intermediate care units. We believe, as it is our experience, that most of patients undergoing NIV were in acute respiratory failure, even though we were not able to confirm it. According to our statistics, the mean number of comorbidities of patients admitted to internal medicine general wards is 13 and mean ACCI is 5.7 points, higher than the results of patients in intermediate care units. As the evidence has proved the safety of NIV in general wards,6 future investigation should approach this reality.
Our work has several limitations. First, our sample is small and some of the comorbidities composing ACCI, such as lymphoproliferative disorders, AIDS, liver disease and metastatic solid cancer, had a low prevalence, limiting conclusions. Mild liver disease and lymphoma seem to increase the risk of 30 and 90 days mortality, respectively, and future studies should evaluate these populations. Second, we included all patients submitted no NIV and future studies should be designed to include more specific populations to uncover differences in the outcomes by main diagnosis. One strength of our study is the high burden of comorbidities in our population that we believe represents better the reality of most practitioners in intermediate and intensive care units nowadays. In fact, most studies lack information about basal performance of extremely ill patients that we frequently find in our clinical practice. Defining the limits between curative and palliative cares is extremely challenging in these patients, and future studies should include more chronically ill individuals. Identification of poor prognostic patients is determinant to reduce unnecessary discomfort, potentially induced by NIV, and to promote earlier end of life care.
To the best of our knowledge no study has previously evaluated the performance of ACCI in predicting outcomes of patients submitted to NIV. We believe that comorbidities have a significant role in the outcomes of patients acutely submitted to NIV but ACCI has several limitations. New prognostic indexes, including comorbidities and independence status, besides acute illness related variables, should be investigated.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors thank the physicians who work at Intermediate Care Unit – Centro Hospitalar do Porto.