The Community-Acquired Pneumonia Organization (CAPO) is an international observational study in 130 hospitals, with a total of 31 countries, to assess the current management of hospitalized patients with community-acquired pneumonia (CAP). Using the centralized database of CAPO was decided to conduct this study with the aim of evaluating the level of adherence with national guidelines in Venezuela, to define in which areas an intervention may be necessary to improve the quality of care of hospitalized patients with CAP.
MethodsIn this observational retrospective study, quality indicators were used to evaluate the management of hospitalized patients with CAP in 8 Venezuelan's centers. The care of the patients was evaluated in the areas of: hospitalization, oxygen therapy, empiric antibiotic therapy, switch therapy, etiological studies, blood cultures indication, and prevention. The compliance was rated as good (>90%), intermediate (60%–90%), or low (<60%).
ResultsA total of 454 patients with CAP were enrolled. The empiric treatment administered within 8h of the patient arrival to the hospital was good (96%), but the rest of the indicators showed a low level of adherence (<60%).
ConclusionWe can say that there are many areas in the management of CAP in Venezuela that are not performed according to the national guidelines of SOVETHORAX.1 In any quality improvement process the first step is to evaluate the difference between what is recommended and what is done in clinical practice. While this study meets this first step, the challenge for the future is to implement the processes necessary to improve the management of CAP in Venezuela.
La Organización de Neumonía adquirida en la Comunidad (CAPO, siglas en inglés: Community Acquired Pneumonia Organization) es un estudio observacional internacional en 130 hospitales de un total de 31 países, para evaluar la gestión actual de los pacientes hospitalizados con neumonía adquirida en la comunidad (NAC). Utilizando la base de datos centralizada de CAPO, se realizó este subestudio con el objetivo de evaluar el grado de cumplimiento con las guías nacionales en Venezuela, para definir en qué áreas se puede intervenir para mejorar la atención del paciente hospitalizado con NAC.
MétodosEn este estudio retrospectivo observacional, se usaron indicadores de calidad para evaluar la atención de pacientes hospitalizados con NAC en 8 centros de Venezuela. El nivel de cumplimiento fue clasificado como óptimo (>90%), intermedio (60–90%), y bajo (<60%).
ResultadosSe enrolaron 454 pacientes con NAC. El tratamiento empírico administrado dentro de las 8 horas de la admisión fue óptimo (96%), el resto de los indicadores mostraron un bajo nivel de cumplimiento (<60%).
ConclusionesPodemos decir que existen muchas áreas en el manejo de las NAC en Venezuela que no se efectúan de acuerdo a las guías nacionales de la SOVETHORAX1. En todo proceso de mejora de calidad la primera etapa es la evaluación de la diferencia entre lo recomendado y lo que se efectúa en la práctica clínica diaria. Este estudio cumple con este primer paso, pero el desafío a futuro es implementar los procesos necesarios para mejorar el manejo de la NAC en Venezuela.
Despite advances in diagnosis and treatment, community-acquired pneumonia (CAP) is still associated with high morbidity and mortality. It is a common cause of outpatient consultation and hospital admission, and has a major impact on healthcare costs. CAP mainly affects the elderly population and patients with multiple comorbidities.3 In Venezuela, it is the ninth cause of mortality nationwide, and is responsible for 3% (3582) of reported deaths, according to data from the “Mortality Yearbook” published by the Venezuelan Ministry of People's Power of Health (MPPH).4
Most CAP guidelines for Latin America follow the general recommendations of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society,3 with only a few exceptions. Since these guidelines are based on scientific evidence, the best results are obtained when they are used in routine clinical practice. The main objective of the guidelines is to improve clinical outcomes and reduce healthcare costs. This, however, can only be achieved by adapting national guidelines to different local settings and evaluating compliance (local implementation).
Levels of compliance with national recommendations can be measured using quality indicators. Quality indicators (also known as a performance indicators) are constructed as percentages, where the numerator represents the number of patients treated in accordance with national guidelines, while the denominator represents the number of times the treatment was indicated.
The Community-Acquired Pneumonia Organization (CAPO) includes investigators from 31 countries and 130 hospitals engaged in a study to evaluate the quality of care delivered to hospitalized patients with CAP.2 Using the CAPO centralized database, we decided to conduct this study to evaluate the level of compliance with national guidelines in Venezuela, and to define areas in which action is needed to improve both the care given to hospitalized patients with CAP and clinical outcomes.
MethodThis is a retrospective, observational sub-study of the core CAPO study in which the medical records of hospitalized patients with CAP in 8 Venezuelan hospitals were evaluated. Hospitals with a researcher actively involved in the CAPO were chosen to participate in the study.
Since the aim of the CAPO study is to evaluate real practice, data on clinical practice were sourced from patient medical records, and cases discharged from hospital were retrospectively reviewed. All medical records with a diagnosis of CAP were included in the study.
Diagnosis of CAP: criteria for making a correct diagnosis of CAP were met if the patient presented with a new (or changing) pulmonary infiltrate associated with at least one of the following: new or increased cough, fever or hypothermia, leukocytosis, left shift, or leukopenia. Pneumonia was considered to be community-acquired if patients had been hospitalized <48h since the onset of symptoms.
As this was a retrospective observational study, no clinical or laboratory protocols were required.
Patients were identified by their initials (first name, second name, and surname) and assigned a code number. Data relating to a series of quality indicators were collected to define the actual management of hospitalized patients with CAP and to evaluate whether the treatment given differed from that recommended in guidelines.
The quality indicators used in the study were devised by the principal investigators and were based on a literature review1,3,5–11 and on CAP management recommended by the ATS and IDSA.3
The study and use of the data collected were approved by the CAPO research committee.2 The clinical records of 454 patients hospitalized between 2002 and 2011 were selected by random sampling, taking the first case of CAP recorded at the start of each month. The patients had been treated in the internal medicine and respiratory medicine departments of 8 tertiary hospitals in Venezuela.
The following quality indicators were evaluated:
Need for hospitalization: the proportion of patients correctly hospitalized according to their mortality risk (CURB-65 ≥2) was used as a quality indicator. For the purpose of this indicator, the numerator was the number of patients hospitalized with risk class ≥2, and the denominator was the total number of hospitalized patients with CAP.
Assessment of oxygenation: this was defined as the proportion of patients with baseline oxygen saturation determined at the time of admission. For the purpose of this indicator, the numerator was the total number of patients with baseline oxygen saturation measured by pulse oximetry and/or arterial blood gas at the time of admission, and the denominator was the total number of hospitalized patients with CAP.
Choice of initial empirical antibiotic therapy: this was defined as the proportion of hospitalized patients treated empirically in accordance with national guidelines. For the purpose of this indicator, the numerator was the number of hospitalized patients treated empirically in accordance with national guidelines, and the denominator was the total number of hospitalized patients with CAP.
Administration of antibiotics within 8h of admission: the proportion of hospitalized patients with CAP given empirical antibiotic therapy within 8h of admission. For the purpose of this indicator, the numerator was the number of hospitalized patients given the first dose of antibiotic therapy within 8h of admission, and the denominator was the total number of hospitalized patients with CAP.
Switch therapy: this was defined as the proportion of patients switched from intravenous to oral antibiotics in accordance with the criteria established in national guidelines. For the purpose of this indicator, the numerator was the number of hospitalized patients with CAP switched to oral antibiotics within 24h of being identified as switch candidates, and the denominator was the total number of hospitalized patients with pneumonia who were candidates for switch therapy.
Etiological study: this was defined as the proportion of patients hospitalized for CAP from whom clinical samples were taken to identify the causal agent (good quality sputum culture, blood culture, pneumococcal and/or legionella urinary antigen test, serology for atypical pathogens, PCR, etc.). For the purpose of this indicator, the numerator was the number of patients hospitalized for CAP from whom at least 1 clinical sample was taken to identify the causal agent, and the denominator was the total number of hospitalized patients with CAP.
Indication for blood culture: this was defined as the proportion of hospitalized patients with CAP with the following clinical criteria: admission to the intensive care unit (ICU), alcohol abuse, severe chronic liver disease, asplenia, leukopenia, pulmonary cavitation on chest radiograph and/or pleural effusion, from whom blood was drawn for culture at admission. These are the clinical criteria for blood culture in CAP patients recommended in national guidelines. For the purpose of this indicator, the numerator was the total number of patients with these clinical criteria from whom blood was drawn for culture at admission, and the denominator was the total number of patients hospitalized for CAP with the abovementioned clinical characteristics.
Prevention of CAP: prevention of CAP was evaluated using 3 quality indicators: (1) patients given pneumococcal vaccine, (2) patients given influenza vaccine, and (3) patients offered smoking cessation therapy.
The proportion of patients given polysaccharide pneumococcal vaccine was studied. For the purpose of this indicator, the numerator was the number of patients given the pneumococcal vaccine, and the denominator was the total number of patients who were candidates for the vaccine.
The proportion of patients given the influenza vaccine was studied. For the purpose of this indicator, the numerator was the number of patients given the influenza vaccine, and the denominator was the total number of patients that were candidates for the vaccine during the influenza season.
The proportion of patients offered smoking cessation therapy was studied. For the purpose of this indicator, the numerator was the number of patients offered smoking cessation therapy, and the denominator was the total number of patients that were smokers and able to understand the explanation.
Level of ComplianceThe level of compliance with national guidelines was classified as optimal when compliance was greater that 90% (no improvement strategies required), intermediate when compliance ranged from 60% to 90% (action required to improve quality to optimal level), and poor when compliance was below 60% (urgent action required to improve quality).
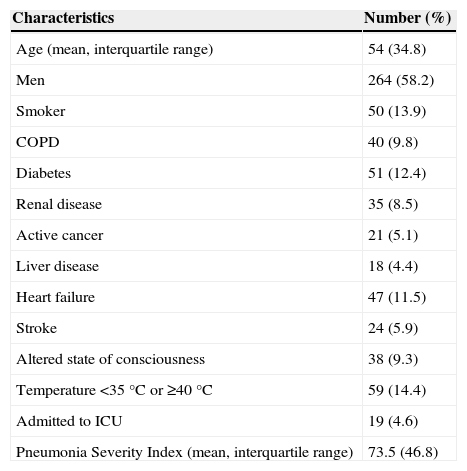
ResultsThe initial approach and clinical treatment given to 454 patients hospitalized in 8 tertiary hospitals in Venezuela were analyzed. Patient demographics, comorbidities and severity of CAP at admission are described in Table 1.
Characteristics of Hospitalized Patients With Community-Acquired Pneumonia (CAP).
| Characteristics | Number (%) |
|---|---|
| Age (mean, interquartile range) | 54 (34.8) |
| Men | 264 (58.2) |
| Smoker | 50 (13.9) |
| COPD | 40 (9.8) |
| Diabetes | 51 (12.4) |
| Renal disease | 35 (8.5) |
| Active cancer | 21 (5.1) |
| Liver disease | 18 (4.4) |
| Heart failure | 47 (11.5) |
| Stroke | 24 (5.9) |
| Altered state of consciousness | 38 (9.3) |
| Temperature <35°C or ≥40°C | 59 (14.4) |
| Admitted to ICU | 19 (4.6) |
| Pneumonia Severity Index (mean, interquartile range) | 73.5 (46.8) |
ICU, intensive care unit.
The results of the quality indicators were as follows:
A total of 361 patients hospitalized for CAP underwent CURB-65 assessment on admission. Of these, only 29 (8%) had a CURB-65 score of ≥2.
In 410 (90%) patients, data relating to oxygenation assessment on admission were recorded. Baseline oxygen saturation by pulse oximetry and/or arterial blood gas was performed in only 62 (15%) of these patients.
Initial empirical antibiotic therapy compliant with national guidelines was recorded in 250 (55%) out of a total of 454 patients hospitalized for CAP. The most commonly used antibiotics were ampicillin/sulbactam, cefotaxime, ceftriaxone, and levofloxacin.
The time of hospital admission and the time of administration of the first dose of antibiotics were recorded in 321 patients. A total of 308 (96%) patients received empirical treatment within 8h of admission.
Out of a population of 454 patients hospitalized for CAP, 258 (57%) were candidates for switch therapy in the first 7 days of hospitalization. Compliance with national guidelines was recorded in 38 (15%) of these patients.
Information regarding clinical samples taken for etiological study was available in 84 patients hospitalized for CAP. Of these, at least 1 clinical sample to determine the etiological agent was taken from 23 (27%) patients.
A total of 98 patients hospitalized for CAP met the clinical criteria for blood culture. Of these, blood samples were drawn from only 23 (23.5%) patients.
As far as prevention of CAP is concerned, compliance with polysaccharide pneumococcal vaccination recommendations was documented in 6% of the study population (18 out of 325 patients). Compliance with influenza vaccination recommendations was documented in 6% of the study population (18 out of 324 patients), and 34% of the population (33 out of 96 patients) were offered smoking cessation therapy.
With regard to the clinical outcome of patients hospitalized for CAP, the mean number of days to clinical stability was 5.5, with a standard deviation of 2.1. Mean hospital stay, meanwhile, was 7.9 days, with a standard deviation of 37.8, and the hospital mortality rate was 34 patients (8.7%).
DiscussionThis study shows that compliance with national guidelines is poor in several aspects of the care given to patients hospitalized for CAP in Venezuela. Areas of poor compliance are: need for hospitalization, assessment of oxygenation, initial empirical antibiotic therapy, switch therapy, etiological study and indication for blood culture, administration of pneumococcal and influenza vaccine, and the offer of smoking cessation therapy.
A number of organizations that audit the quality of care in hospitals are increasingly using quality indicators as an evaluation tool.12
Since no previous studies evaluating adherence to national CAP management guidelines in Venezuela have been performed, we had no point of reference with which to compare our findings. A similar study, also using the CAPO database, was conducted in Argentina in 2003,6 reporting poor compliance with recommendations on switch therapy, administration of pneumococcal vaccine and offer of smoking cessation therapy. Considering that these countries share certain characteristics and social and economic values, it is interesting to note the difference in compliance with the remaining quality indicators used in both studies. One reason for this could be Argentina's tendency to adhere more strictly to care standards defined in European and US guidelines.
As far as the need for hospitalization is concerned, the IDSA/ATS clinical practice guidelines for management of CAP in adults published in 20073 recommend the use of the CURB-65 severity score as the criterion for hospitalization (Level I evidence). They clarify, however, that the attending physician can also take other factors into consideration, such as the availability of and due compliance with oral antibiotic therapy, and the availability of social and family outpatient support resources. Other factors that can influence the decision to hospitalize the patient are decompensation of underlying diseases (for example, cardiovascular disease, COPD, etc.) and poor response to oral antibiotic therapy in the outpatient setting. Alberti et al., in a study conducted in Italy, reported that 58% of patients with a CURB-65 score of 0–1 seen in emergency rooms were hospitalized, with admission justified in 83% of these patients.13 Another important factor to consider in hospitalization is hypoxemia. Although this is not one of the CURB-65 parameters, it has been identified by some authors in almost 50% of patients with a CURB-65 score of 0–1, and also as the main reason for hospitalization.14 Nevertheless, it is important to note that compliance with the recommendation to assess baseline oxygen saturation was poor in Venezuela. This factor, therefore, was unlikely to have had any major influence on the decision to hospitalize in our cohort of CAP patients. The same problem arises with the “confusion” parameter: a patient with CAP combined with mental confusion would probably be hospitalized even with a score of 1. These elements, and above all social factors, could well be among the reasons for hospitalization in 92% of patients with a CURB-65 score <2 in our cohort.
Assessment of oxygenation at admission is part of good clinical practice. It is used to determine the severity of CAP and helps identify the need for hospitalization (even though it is not a CURB-65 parameter). It is also a determining factor in the decision to give oxygen therapy and transfer the patient to the ICU. Given the importance of this assessment, it is alarming to note that it was carried out in only 15% of patients admitted for CAP, particularly since it is an economic, quick, simple diagnostic test that does not need to be performed by highly trained medical personnel.
It is clear that the early administration of antibiotic therapy does not always imply that the right drug was given. The use of broad-spectrum antimicrobials could be an indication of failure to identify the source of infection. Compliance with the recommendation to administer the first dose of antibiotics within 8h of admission was good in our cohort. However, there is no consensus in the literature as to the best time to administer antibiotics in patients hospitalized for CAP and the impact of this on clinical outcomes.15–22
Although antibiotics were generally administered at an early stage, adherence to national guideline recommendations on the choice of antibiotic was poor. Despite availability of the antibiotics most commonly used in CAP, most physicians opted for beta-lactam monotherapy without considering coverage for atypical pathogens. The literature has shown that combination therapy in CAP patients improves clinical outcomes.23,24 A possible explanation for these shortcomings could be the failure to implement local guidelines on antibiotic therapy and the rational use of antibiotics, and poorly trained healthcare personnel. Another factor could be logistics, i.e., lack of coordination between the emergency department, the ward and the hospital pharmacy.
Failure to comply with recommendations on switch therapy, meanwhile, is common to all countries worldwide.6,25–27 The same factors preventing medical personnel from correctly implementing switch therapy in CAP patients found in Argentina and Venezuela have also been reported in other countries. Some of the reasons for this could be the lack of clearly stated recommendations in local clinical practice guidelines, the physician's misgivings regarding the outcome of the switch, and absence of protocols to check for switch criteria during daily ward rounds (for example, check lists in medical records). According to the literature, compliance with this indicator ranges from 22% to 94%.25 In this respect, it should be noted that an early switch to oral therapy is extremely beneficial: it shortens hospital stays and simplifies initial empirical antibiotic therapy while reducing both the detrimental effects of broad-spectrum antibiotics (infections caused by catheters, Clostridium difficile, colonization by multi-drug resistant pathogens, etc.) and hospital costs.
The level of compliance with each indicator is obviously not an isolated factor; rather, each indicator is connected to the following, like links in a chain. Therefore, it is far more difficult to switch from intravenous to oral therapy if the etiological agent of the disease is unknown. As a result, empirical intravenous therapy is often continued for the entire course of treatment, with the subsequent increase in care costs, length of stay, and adverse effects.
As mentioned above, it is essential to determine the etiological agent of CAP in order to improve compliance with other quality of care indicators. These diagnostic tests contribute to early implementation of switch therapy and reduce the risks associated with intravenous catheters and broad-spectrum antibiotics. The lack of etiological studies in hospitalized patients with CAP in Venezuela is alarming. Various factors could contribute to this situation: restricted access to diagnostic kits due to high cost (pneumococcal and/or legionella urinary antigen test), failure to understand that early etiological diagnosis can improve other indicators, lack of training in sampling techniques (medical staff), infrastructural shortcomings and shortage of laboratory technicians trained in the interpretation of test results, and logistics problems (lack of coordination between the emergency department, the ward and the hospital pharmacy prevents clinical samples from being correctly taken and transported to the lab).
Prevention strategies are probably poorly implemented in most Latin American countries, and therefore poor compliance with vaccination and smoking cessation recommendations in both Argentina and Venezuela are to be expected. Studies in other parts of the world have found a similar trend: only 17% of smokers in internal medicine wards and 66% in cardiology wards are advised to stop smoking.28 Some authors have suggested that advice received in hospital does not result in smoking cessation if it is not followed-up for at least 1 month after discharge.29 There are several pharmacological aids for cessation (nicotine patches, varenicline, ibupropion, etc.) that are often ignored in these countries for reasons of cost and because many believe that only behavioral strategies are effective in achieving smoking cessation.
Pneumococcal vaccination still plays an important part in efforts to reduce the severity and complications associated with invasive pneumococcal disease, above all in elderly patients.30 The reasons for poor compliance with this parameter could be similar in both countries. The most common reasons include the lack of implementation of local guidelines and vaccination algorithms, scarcity of vaccines and trained healthcare professionals, lack of electronic clinical records that would improve registry practices, and lack of inclusion criteria check lists in clinical records, to name just a few.
We believe that these 3 prevention indicators can be improved by improving registry techniques and clinical record documentation. Such a low level of compliance is more likely to be related to under-reporting than non-compliance.
This study has a number of important limitations. First, we did not consecutively evaluate all hospitalized patients with CAP. We acknowledge that a random selection of patients (the first case documented each month) is not the best approach, and could have biased the selection to a certain extent. This could also explain why most cases of pneumonia in our cohort were not severe. Secondly, study sites (hospitals) were chosen on the basis of the active participation of one of the researchers in the CAPO, which could suggest that our findings are not a true reflection of the reality of Venezuela.
The major strength of this study is that this is the first collection of detailed information on hospitalized patients with CAP in Venezuela, including all the quality indicators discussed in the literature.1,3,5–11
In conclusion, our findings show that there are many aspects of CAP management in Venezuela that do not comply with national SOVETHORAX guidelines.1 In any quality improvement strategy, the first step is always to evaluate differences between recommendations and routine clinical practice This study has taken the first step; the challenge now is to implement the processes needed to improve CAP management in Venezuela.
Contributors- •
Gur Levy: study protocol, concept, study design. Quality evaluation and interpretation of data. Writing. Critical review of the manuscript and approval of the final version.
- •
Mario Pérez: study protocol, concept, study design. Quality evaluation and interpretation of data. Writing. Critical review of the manuscript and approval of the final version.
- •
Benito Rodríguez: study protocol, concept, study design. Quality evaluation and interpretation of data. Writing. Critical review of the manuscript and approval of the final version.
- •
Ana Hernández Voth: study protocol, concept, study design. Quality evaluation and interpretation of data. Writing. Critical review of the manuscript and approval of the final version.
- •
Jorge Pérez: quality evaluation and interpretation of data. Writing. Critical review of the manuscript and approval of the final version.
- •
Martin Gnoni: quality evaluation and interpretation of data. Writing. Critical review of the manuscript and approval of the final version.
- •
Robert Kelley: study protocol, concept, study design. Statistical analysis. Database. Quality evaluation and interpretation of data. Critical review of the manuscript and approval of the final version.
- •
Timothy Wiemken: study protocol, concept, study design. Statistical analysis. Quality evaluation and interpretation of data. Critical review of the manuscript and approval of the final version.
- •
Julio Ramirez: study protocol, concept, study design. Statistical analysis. Database. Quality evaluation and interpretation of data. Writing. Critical review of the manuscript and approval of the final version.
The authors declare they have no conflicts of interest.
We would like to thank the members of the research team who helped collect the data for this study.
Please cite this article as: Levy G, Perez M, Rodríguez B, Hernández Voth A, Perez J, Gnoni M, et al. Cumplimiento con las guías nacionales en pacientes hospitalizados con neumonía adquirida en la comunidad: resultados del Estudio Capo en Venezuela. Arch Bronconeumol. 2015;51:163–168.