The accuracy of auto-CPAP devices in determining residual apnea–hypopnea index (AHI) has been evaluated in several studies1–7 but has not been confirmed in adaptive servo-ventilation (ASV) equipment. However, these data inform treatment and can significantly affect whether respiratory events are being treated optimally with the prescribed pressure setting.
We assessed the accuracy of respiratory event detection by ASV devices in 7 patients with central apneas/Cheyne Stokes respiration (CSA/CSR) and 9 with complex sleep apnea syndrome (CompSAS), diagnosed with respiratory polygraphy (RP) or conventional PSG. CSA was defined as an AHI >15 with predominant (>50%) central apneas according to AASM 2007 criteria.8 CompSAS was defined as the appearance of central apneas (CAI of >15/h), during CPAP titration9 in patients with obstructive sleep apnea at baseline, which persisted at follow-up using CPAP.
All patients were offered ASV treatment. The device brand depended on the supplier used by the Catalan Health Service, who provided treatment free of charge. The AutoSet CS, which did not allow for automatic expiratory pressure (EPAP) adjustment, was set at EPAP 6cm H2O, minimum pressure support (PS) 3cm H2O, maximum pressure (P max) 25cm H2O. The settings for the BiPAP autoSV Advanced were: EPAP min 4cm H2O, EPAP max 10cm H2O, PS min 0cm H2O and PS max 25cm H2O.
A PSG with the patient's ASV device was performed 3 months later, and the AHI obtained from the device's software analysis (ASV-AHI) was compared to the AHI manually scored from PSG (PSG-AHI) over total sleep time (PSG-AHI-TST) and recording time (PSG-AHI-RT). Leaks obtained from the ASV smart card were recorded for analysis.
The agreement between PSG-AHI and ASV-AHI was studied with a Bland and Altman plot.10 A Friedman correlation was used to assess any association between mask leakage and difference between PSG-AHI and ASV-AHI.
Values were expressed as median (25th–75th percentile), or mean±SD.
Fifteen patients were men (93.7%), with no significant differences in baseline parameters between subjects with CSA or CompSAS in terms of age (67 [63–79] years vs. 73 [63–78] years), body mass index (28 [25–32]kg/m2), Epworth score (4 [3–6] vs. 6 [4–13]) or AHI (50 [48–81] vs. 46 [41.5–60.5]).
Among the 16 patients, 2 declined ASV treatment. Comp SAS patients, who had been previously treated with CPAP for 284 days (34–902), were treated with a BiPAP autoSV Advanced (n=2) or AutoSet CS (n=6), and patients with CSA/CSR with a BiPAP autoSV Advanced (n=2) or AutoSet CS (n=4).
PSG at 3 months with the patient's device showed resolution of CA. In 2 patients, downloading from the ASV device was not possible (1 BiPAP autoSV Advanced, 1 AutoSet CS).
ASV-AHI in the 12 patients (3.3 [1.4–4.3]) was significantly lower than AHI-TST or AHI-RT (10.6 [4.8–20.2], P=.005 and 7.5 [3.8–15.7], P=.008, respectively). HI derived from the ASV device was also significantly lower than PSG HI-TST (2.8 [1.2–3.9] vs. 9.5 [3–20], P=.005) and PSG HI-RT (7.4 [2.5–15.5], P=.010).
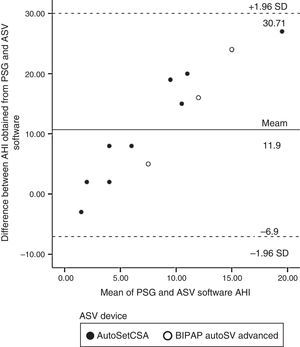
Fig. 1 shows the Bland and Altman plot of the difference between PSG-AHI and ASV-AHI against the mean of both measurements, with a mean difference of 11.9±9.6 (95% limits of agreement −6.90, 30.71), which was greater because the residual AHI was higher.
The PSG-AHI was lower than 10events/h in 6 of the 14 patients (42.8%) and lower than 15 in 8 of the 14 patients (57.1%), whereas according to ASV-AHI, all patients showed an AHI lower than 10.
No significant correlation was observed between mask leaks and the mean difference between PSG-AHI and smart card ASV-AHI (r=−0.423, P=.256) in patients using the AutoSet CS device. No high leaks were observed in patients using BiPAP autoSV Advanced.
The accuracy of internal software in estimating residual AHI has been addressed in a small number of studies with auto-CPAP devices1–7 but, to our knowledge, there is no published information on ASV devices. While the ASV device adequately treated respiratory events in all patients according to the device software, treatment was sub-optimal in a substantial number of patients, according to PSG scoring. Thus, the ASV software underestimated the AHI, at the expense of underscoring hypopneas. Inter-observer variability in PSG manual scoring, especially in identifying hypopneas and the different criteria used to score hypopneas, have been suggested as possible explanations for the variance in results obtained by different studies analyzing auto-CPAP devices.1,7 In our study, the same physician manually scored respiratory events on ASV treatment. Differences in hypopnea scoring criteria between human and machine detection of residual events are a more likely explanation for this finding. Manual hypopnea scoring criteria was a 50% airflow reduction followed by at least 3% desaturation and/or an arousal,8 whereas the ASV device software does not take into account oxygen saturation or arousals from sleep when detecting respiratory events. Differences between PSG sleep time and recording time could suggest another explanation. Nonetheless, ASV-AHI was significantly lower than PSG-AHI whether calculated according to the sleep efficiency or based on recording time. Finally, the underscoring of residual events by the ASV software could also suggest the presence of excessive leaks, although we did not find any significant correlation between mask leaks during PSG as obtained by the ASV device and the difference between PSH-AHI and smart card ASV_AHI.
Although we studied 2 different populations of patients with CA and 2 different ASV devices, the baseline characteristics of both groups did not differ, and the effect of both ASV devices on CA was identified in both CSA and CompSAS subjects. However, due to the small number of patients using each device, we were unable to compare both devices in terms of analysis software accuracy.
In summary, device software-derived AHI significantly underestimated manually scored AHI at PSG, with a mean bias of 11, at the expense of hypopneas, and to a greater extent at a higher residual AHI. The clinical significance of residual AHI in these patients, many of whom suffer from heart failure, is still unknown. Further studies are needed to confirm these results and whether they are true of any ASV device.
Author's ContributionMaría Guadalupe Silveira: data analysis, manuscript writing Gabriel Sampol: study design, manuscript review Roser Cambrodi: data collection Àlex Ferre: data collection Patricia Lloberes: study design, manuscript writing, supervision.
The authors wish to thank Yvette Jusseaume for the English translation of the manuscript and Rosa Llòria for her help in the editorial assistance.
Please cite this article as: Silveira M-G, Sampol G, Cambrodi R, Ferre À, Lloberes P. Software de los dispositivos de servoventilación adaptativa para la evaluación de los episodios respiratorios residuales de pacientes con apneas centrales o complejas. Arch Bronconeumol. 2017;53:455–457.