Pulmonary lymphangitic carcinomatosis is associated with poor prognosis and tissue collection is difficult depending on the respiratory condition in such patients.1 Liquid biopsy could be highly effective and help in addressing the problem of poor prognosis in lymphangitic carcinomatosis patients. Here, we report a case of lung adenocarcinoma with acute severe respiratory failure caused by pulmonary lymphangitic carcinomatosis at initial diagnosis, who could receive appropriate therapy by using liquid biopsy.
A 70-year-old man with a 30 pack-year history of smoking was referred to our hospital with a 1-month history of slight fever and dyspnea on exertion. Initial diagnosis at a previous hospital had indicated bacterial pneumonia accompanied by interstitial pneumonia, which had been treated with an antibacterial drug. Sputum cytology had revealed adenocarcinoma and the patient was referred to our hospital.
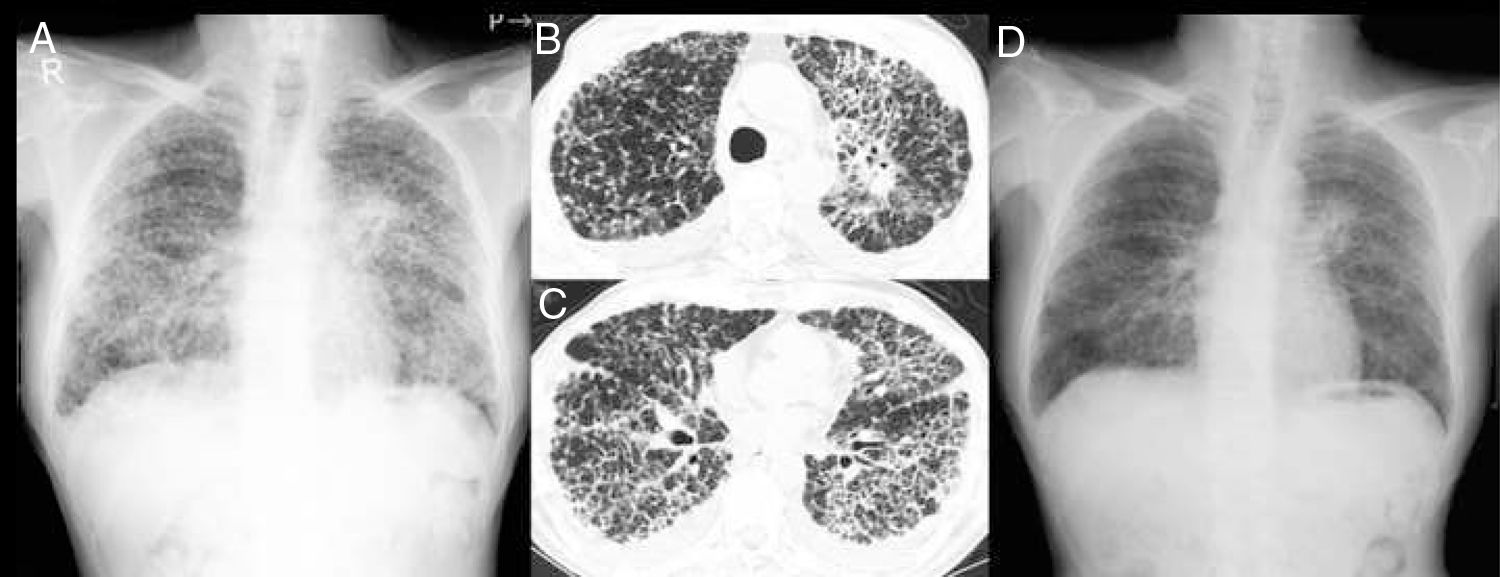
On admission, the patient had bilateral inspiratory crackles on chest auscultation. Laboratory findings showed severe hypoxemia and elevated levels of Krebs von den Lungen-6 (KL-6, 2638U/mL) and carcinoembryonic antigen (CEA, 6.9ng/mL). Chest X-ray showed a mass shadow in the left hilar region and bilateral diffuse interstitial opacities (Fig. 1A). Computed tomography (CT) imaging revealed a mass in the left upper pulmonary lobe, bilateral diffuse beaded thickening of the intralobular septum, and minor bilateral pleural effusions (Fig. 1 B and C). Performing bronchoscopy was considered difficult because high flow of oxygen (reservoir mask: 6L/min) was required for severe respiratory failure.
Chest X-ray showing a mass shadow in the left hilar region and bilateral diffuse interstitial opacities (A). Computed tomography revealing a mass in the left upper pulmonary lobe (B), pulmonary carcinomatosis lymphangitis, and minor bilateral pleural effusions (C). Chest X-ray showing marked improvement after four weeks of treatment (D).
Adenocarcinoma was confirmed by cytological examination of the pleural effusions obtained by left pleural puncture, and epidermal growth factor receptor (EGFR) exon 19 deletion was found in the plasma EGFR gene mutation test at the time of hospitalization. The patient was confirmed for EGFR mutation-positive lung adenocarcinoma accompanied by carcinomatosis lymphangitis and pleurisy, and treatment with osimertinib (80mg/day) was initiated on the fourth day of admission.
Although respiratory failure exacerbated and noninvasive positive pressure ventilation (NPPV) was required temporarily, respiratory failure resolved and NPPV treatment was withdrawn after two weeks. After four weeks of treatment, the patient experienced symptomatic relief as evidenced by marked improvement in the patient's X-ray (Fig. 1D). EGFR exon 19 deletion was also detected in the pleural effusion samples. The patient was returned to the previous hospital. Currently, the patient is in partial remission following 12 months of treatment with osimertinib.
Pulmonary lymphangitic carcinomatosis is mainly detected in patients with adenocarcinoma, especially those with lung, breast, and gastric cancers, and associated with poor prognosis.1 In advanced non-small cell lung cancer (NSCLC), identifying driver gene mutations is recommended, but tissue collection is difficult depending on the general condition of the patient, such as respiratory failure, which is often a time-limited situation in such patients. Liquid biopsy is useful when tissue sampling is difficult or inadequate.2 Although liquid biopsy can only currently be used in clinical practice for EGFR sensitizing and resistance mutations, various NSCLC driver genes have been detected successfully with the help of several plasma-based tests including molecular tests (such as detection of ROS-1) even before proceeding to tissue-based tests.3–6
In the present case, the time gap between the plasma EGFR mutation test and result report was only three days. This case indicates that liquid biopsy for detecting other driver gene mutations in addition to the EGFR mutation are warranted, since a therapeutic effect can be expected in the patient with driver gene mutation-positive NSCLC; this is true even in cases where tissue tests are difficult, such as in patients with lymphangitic carcinomatosis, which generally have poor prognosis.
In summary, we reported a case of EGFR mutation positive lung adenocarcinoma with acute severe respiratory failure caused by pulmonary lymphangitic carcinomatosis. Although tissue collection was difficult, the patient could receive appropriate therapy by using liquid biopsy. Liquid biopsy could help in addressing the problem of poor prognosis in lymphangitic carcinomatosis patients.