Immuno-oncology has led to a revolution in the treatment of certain cancers, and is proving beneficial in an increasing range of tumor types. Immune checkpoint inhibitors (ICIs), such as monoclonal antibodies that target the regulatory protein PD-1, stimulate the immune system by shoring up the body's defense against tumor cells.1 However, these compounds are associated with an increase in immune reactions against the host tissue, causing adverse effects, not least in the respiratory system.
We report the case of a patient with metastatic melanoma who developed acute fibrinous and organizing pneumonia (AFOP) associated with pembrolizumab, an adverse effect not previously described in the literature.
Our patient was a 61-year-old man, who quit smoking 10 years previously with a pack-year index of 90, and who presented no respiratory history. He was diagnosed with ulcerated nodular melanoma in the scapular region, which was resected with extended margins in October 2019. In October 2020, he started treatment with pembrolizumab due to subcutaneous progression.
Seven months after treatment with pembrolizumab, he presented in the emergency department with dry cough, a 1-month history of dyspnea worsening until occurring on minimal exertion, and oppressive chest tightness. He was afebrile, breathing normally, with SatO2 94% and incipient signs of fingernail clubbing. Lung auscultation revealed bilateral crackles.
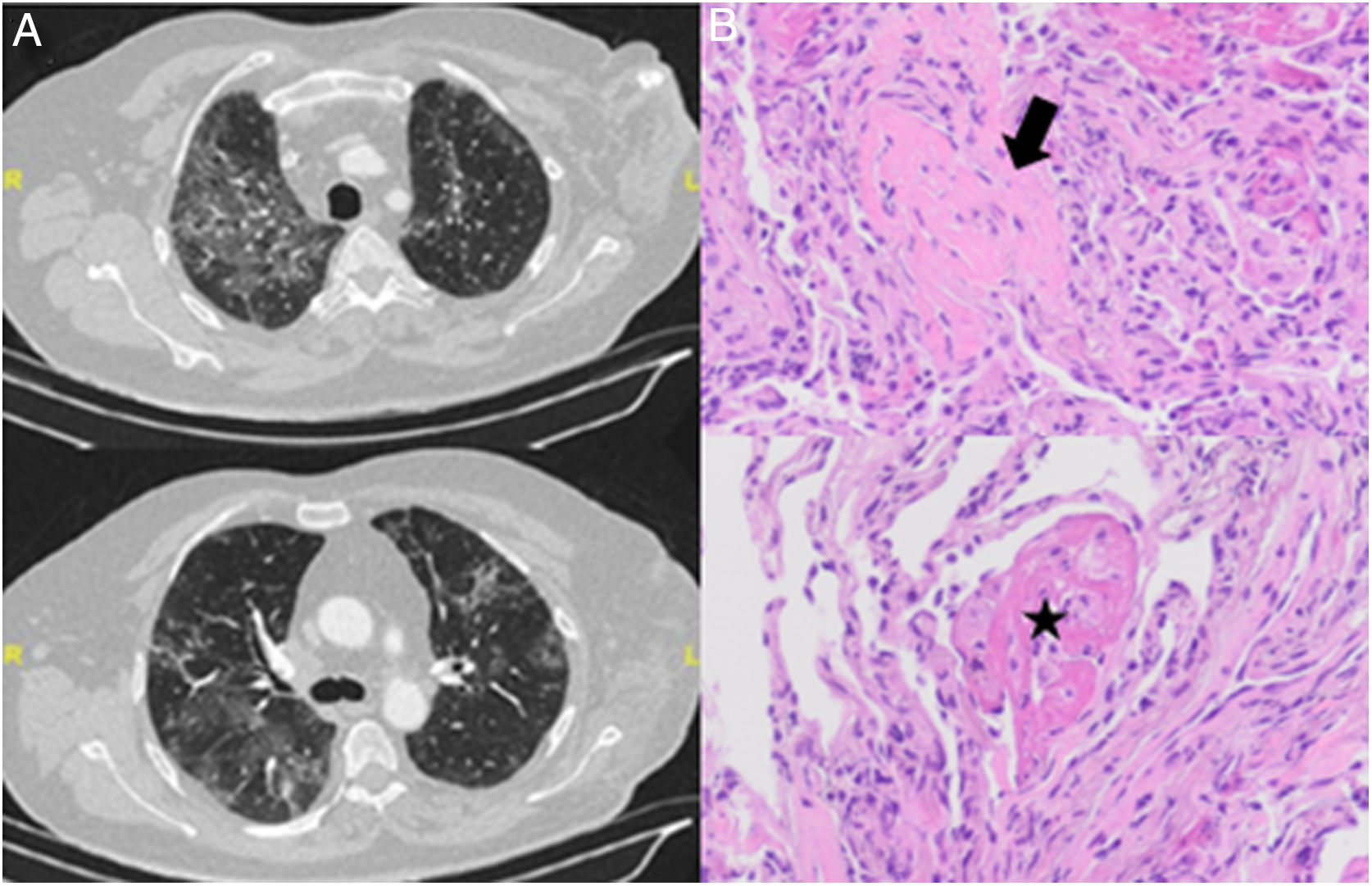
Chest X-ray showed a bilateral interstitial pattern predominantly in the middle and lower fields. Computed tomography (CT) revealed extensive bilateral pulmonary infiltrates with new signs of ground glass appearance (Fig. 1A).
(A) Chest CT slices showing bilateral ground-glass infiltrates. (B) Transbronchial biopsy with hematoxylin–eosin staining showing organizing pneumonia with Masson bodies (arrow) and organizing fibrin aggregates (“balls”) (star). Note the absence of hyaline membranes, and type II pneumocyte hyperplasia and mild interstitial inflammatory infiltrate.
Clinical laboratory tests showed no significant changes, and specific IgG was normal. Spirometry showed mild restriction (forced vital capacity 78%) and slight decrease in diffusion (lung diffusion capacity for carbon monoxide 65%).
Fiberoptic bronchoscopy was performed with bronchoalveolar lavage and aspiration, revealing no evidence of malignancy or opportunistic infection. Lymphocytic alveolitis was detected with a CD3 count of 51% and CD4/CD8 ratio of 0.36. The histology study of the transbronchial biopsy identified a pattern of organizing pneumonia with AFOP foci (Fig. 1B).
Treatment with methylprednisolone 1mg/kg began and immunotherapy was discontinued with good clinical response. The patient was discharged with a tapering prednisone regimen. A follow-up high-resolution computed tomography scan at 1 month showed almost complete disappearance of the pulmonary opacities.
Respiratory adverse effects attributed to the use of ICIs include organizing pneumonia (OP), diffuse alveolar damage (DAD), sarcoidosis-like granulomatous reactions, eosinophilic pneumonia (EP), and AFOP.2
Histologically, AFOP is characterized by patches of intra-alveolar fibrin balls and organizing pneumonia. It is differentiated from OP by the absence of hyaline membranes and fibrin deposits, and from DAD that presents with both fibrin and hyaline membranes. EP, on the other hand, is characterized by elevated eosinophil levels.3
Only 10.6% cases of AFOP are drug-related, most commonly with abacavir or amiodarone,4 while only a single case has been described with ICIs (nivolumab).5
In conclusion, it is important to monitor these adverse effects, which are becoming more frequent due to the increased use of ICIs. Moreover, biopsy is essential for the diagnosis of AFOP, as very few cases associated with the use of ICIs have been reported. This is the first report of a pembrolizumab-related event published to date.
Conflict of interestsThe authors state that they have no conflict of interests.