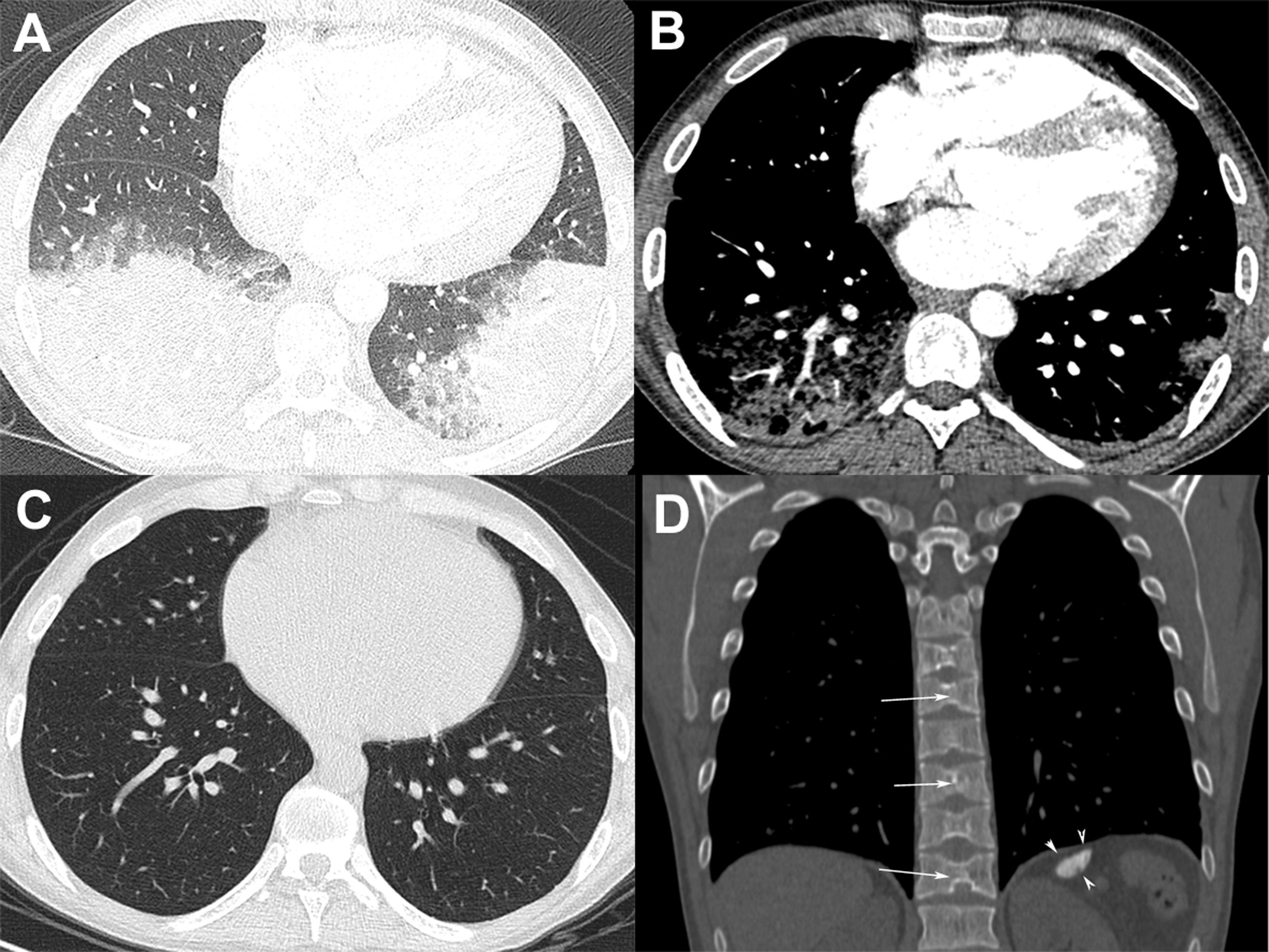
A 24-year-old man with sickle cell disease was admitted to our emergency department due to high fever (39.4°C) and bilateral pleuritic chest pain that had started 5 days previously. He had been admitted two times in the previous month due to pain crisis and respiratory symptoms. At that time, a chest X-ray showed a consolidation in the left lower lobe, and bacterial pneumonia was diagnosed. Pulmonary auscultation revealed decreased breath sounds over the lower lung fields. Laboratory tests showed mild leukocytosis (white cell count, 14,100/cm3) with 64% neutrophils, anemia (hemoglobin level, 7g/dl), thrombocytosis (790,000 platelets), and elevated C-reactive protein (98mg/L) and lactate dehydrogenase levels (482U/L). The patient underwent chest computed tomography (CT) angiography, which demonstrated bilateral pulmonary opacities associated with ground-glass attenuation over the lower lung fields (Fig. 1A) and mild pleural effusion on the right side. Pulmonary thromboembolism was ruled out (Fig. 1B). Two blood culture sets were negative and bronchoscopy was performed; cultures (bacterial and fungal) and GeneXpert results from bronchoalveolar lavage were negative. The patient was diagnosed with acute chest syndrome (ACS) and was treated successfully with blood transfusion, aggressive crystalloid hydration, analgesia, and antibiotic therapy with ampicillin plus sulbactam. His laboratory test values improved to normal and he was discharged from the hospital 7 days after initial treatment with an oral hydroxyurea prescription. One month after discharge, the patient underwent chest CT, which showed complete resolution of the pulmonary opacities (Fig. 1C). CT also showed characteristic findings of sickle cell disease, such as a small and calcified spleen and H-shaped vertebral bodies secondary to bone infarction of the central endplates (Fig. 1D). The final diagnosis of the condition leading to the patient's hospitalization was ACS.
(A) Chest CT image obtained with the lung window setting shows consolidations and ground-glass opacities in both lower lobes. (B) Angio-CT demonstrated no filling defect in the pulmonary arterial system. (C) Chest CT image obtained 1 month after discharge shows complete resolution of the pulmonary opacities. (D) Coronal reconstruction with the bone window setting demonstrates a small and calcified spleen (arrowheads) and H-shaped vertebral bodies with central endplate depressions (arrows).
ACS in patients with sickle cell disease is defined as an acute illness characterized by fever and/or respiratory symptoms (chest pain, dyspnea, or tachypnea), accompanied by the appearance of new pulmonary opacities on imaging examinations.1,2 This condition is most commonly associated with homozygous sickle cell disease and rarely with heterozygous sickle cell disease.2 ACS is the leading cause of hospitalization and death among patients with sickle cell disease.3–5 An exceedingly rare, yet often fatal, complication of ACS is rapid progression to acute respiratory distress syndrome.2 Three major underlying mechanisms of ACS have been proposed. Infection with an excessive inflammatory response to lung injury is the most frequent cause.3–5 Infectious agents associated with ACS in children, in decreasing order of frequency, include viruses, mycoplasma, chlamydia, and bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and others). Fungal infections are uncommon.4 Another proposed mechanism is that the sickled cells cause vascular occlusion and pulmonary infarction. Lastly, fat embolism syndrome may occur as a result of contents released during marrow necrosis, which eventually lodge in the lung and cause pulmonary hypertension and hypoxemia.6 The most frequent pulmonary imaging finding is consolidation, frequently affecting the lower lobe (right more often than left) in adult patients and the middle lobe in pediatric patients. In addition, pleural effusion is equally common in both age groups.5 Ground-glass opacities occur less frequently and can have a more homogeneous distribution.6 Extrapulmonary findings are more prominent in adult patients.5 The differential diagnosis may be challenging because ACS may clinically and radiologically mimic other conditions, such as pneumonia, pulmonary edema, transfusion-related acute lung injury, pulmonary infarction, and atelectasis.1,2 To diminish the vicious cycle of sickle cell hemoglobin polymerization and sickling of erythrocytes, the standard treatment is based on hydration and blood transfusion.1,3,7 The other critical treatment component is antibiotic therapy, as infection is a potential ACS trigger.1,3,8 The correct identification of ACS using clinical and radiological data and its proper treatment are very important, as the rate of mortality due to this syndrome is estimated to be 10%.1,3