Abernethy malformation is rare congenital extrahepatic portosystemic shunt that allows blood from the gut and spleen to reach the systemic venous circulation bypassing the liver filter. This situation leads in some cases to serious complications such as hepatopulmonary syndrome, portopulmonary hypertension, or hepatic encephalopathy. Specifically, hepatopulmonary syndrome may present with chronic hypoxemia. Even though it is a rare condition, extrathoracic pathology should be seek after ruling out cardiac and primary pulmonary disease.
A five year-old boy was admitted for an acute bronchitis and hypoxemia. On physical exam, perioral cyanosis and digital clubbing were discovered. The child had a past medical history of recurrent bronchitis and was followed in the pediatric neurology outpatient clinic under the suspicion of an autistic disorder. In the emergency department nebulized salbutamol was initiated and respiratory symptoms quickly subsided. Three days later, the child improved his clinical condition presenting no dyspnea and a clear cardiopulmonary auscultation but hypoxemia persisted. Orthodeoxia was observed with transcutaneous oxygen saturation decreasing from 88% to 81–82% when he changed from supine to a sitting position. Blood tests showed normal hematocrit (39%), normal liver function, and normal ammonia levels.
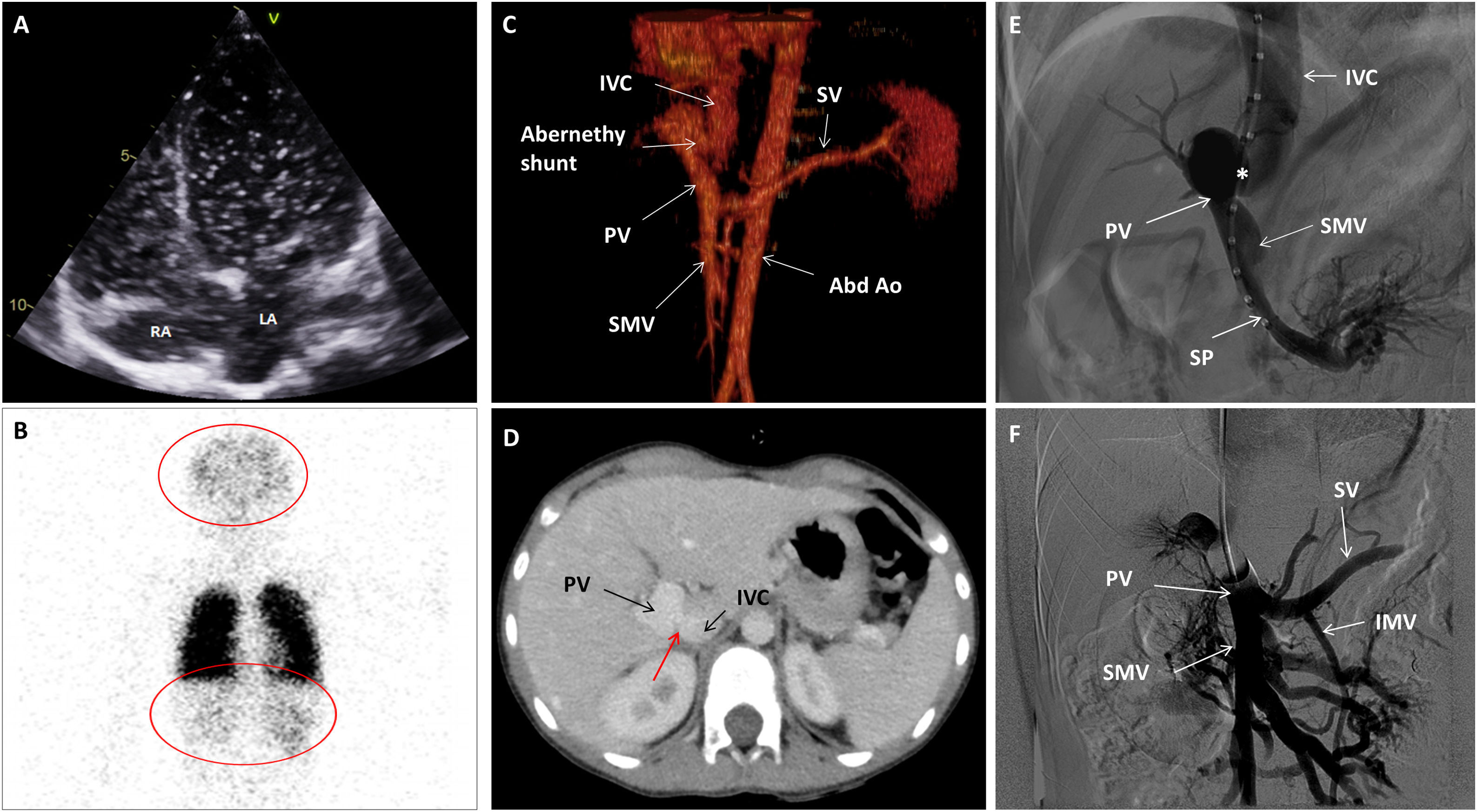
Some tests were performed to find the cause of hypoxemia. No evidence of pulmonary hypertension nor of intracardiac shunts were noticed on echocardiography with agitated serum, but quick pass of microbubbles to the left atrium was suggestive of an intrapulmonary shunt (Fig. 1A, Video 1). No lung disease and no evidence of macroscopic pulmonary arterio-venous fistulas were detected in the thorax angio Computed Tomography (CT). However, under the suspicion of an extra-cardiac right to left shunt, a lung nuclear scanning with Technetium 99m-labeled macroaggregated albumin was requested. Brain and kidney radiotracer uptake confirmed the presence of a right to left shunt of 36% (Fig. 1B). These findings were indicative of an intrapulmonary shunt suggestive of hepatopulmonary syndrome. An abdominal echography demonstrated a porto-caval latero-lateral shunt with a well-developed portal vein system. An abdominal angio-CT confirmed the diagnosis of Abernethy type-2 malformation (Fig. 1C and D).
(A) Echocardiography. Four chamber view depicting a shaked serum. After three cardiac cycles the left atrium was also opacified. (B) Nuclear scanning with Technetium 99m macro-aggregated albumin. A part from the lungs, brain and kidney also showed radioactivity due to tracing uptake (red circles). (C) CT Angiogram. 3D Volume rendering showing the Abernethy malformation. (D) CT Angiogram: Axial plane illustrating the Abernethy shunt (red arrow). (E) Fluoroscopy. Left oblique projection. The centimetered catheter reaches the portal vein from the inferior vena cava, through Abernethy shunt (asterisk). (F) Fluoroscopy. Antero-posterior projection. Balloon shunt occlusion test. Abbreviations: Abd Ao: abdominal aorta. IMS: inferior mesenteric vein. IVC: inferior vena cava. LA: left atrium. PV: portal vein. RA: right atrium. SMV: superior mesenteric vein. SP: splenic vein.
The case was presented to an interventional radiology team of the reference hospital. A catheterization procedure documented a 10mm side-to-side shunt between the portal vein and the inferior vena cava (IVC) (Fig. 1E). The basal portal vein and IVC pressures were 9 and 3mmHg, respectively, and a balloon shunt occlusion test evidenced a moderate increase in portal pressure up to 23mmHg (Fig. 1F). The anatomic (broad and short) shunt morphology was not suitable for any endovascular device. Therefore, the patient was presented to the pediatric hepato-digestive surgery team and underwent a surgical shunt closure through laparoscopy using a hem-o-lok® clip system. Thereafter the patient followed a favorable clinical course. Liver function and liver blood flow were monitored daily through blood test and echography, and remained normal. Before patient discharge, hypoxemia persisted with a basal transcutaneous hemoglobin saturation of 90% and, hence, a home oxygen therapy with nasal cannula (3L/min) was provided. During follow-up the patient experienced a progressive improvement of his hypoxemia and six months after shunt closure oxygen therapy was withdrawn.
Abernethy malformation consists in a rare congenital extrahepatic portosystemic shunt which allows blood from the gut and spleen to reach the systemic venous circulation bypassing the liver.1–6 It was first described in 1793 by the London surgeon John Abernethy who observed for the first time a congenital absence of the portal vein with a mesenteric-caval shunt.3,5,7,8 The estimated incidence of the Abernethy malformation is reported to be 1/30,000 live births in countries where screening for galactosemia is routinely performed.1 This shunt may be classified in two main types: type 1, in which the portal blood is completely diverted into the IVC through a side-to-end anastomosis and where there is an absence or a vague remnant of intrahepatic portal vein system; and type 2, in which the portal system is hypoplastic but patent and communicated side-to-side with systemic veins, usually the IVC.1–6,8–11
Pathophysiologically, Abernethy malformation could be considered as an infrequent cause of hepatopulmonary syndrome which is characterized by a deficient arterial oxygenation due to pulmonary capillary dilatation in the context of liver disease.4,11–14 Blood coming from the gut via superior mesenteric vein and spleen via splenic vein is deviated partially or totally to the IVC. This bypass could imply an imbalance between vasodilator and vasoconstrictor constituents that may lead to vasodilation of pulmonary capillaries, allowing a direct mixed venous blood shunt to the pulmonary veins without being oxygenated thereby causing subsequent hypoxemia.1,4,12,14
Abernethy malformation in children may present a broad clinical spectrum. While some patients can be asymptomatic, others may exhibit marked cyanosis, severe hypoxemia, pulmonary arterial hypertension, digital clubbing, vascular anomalies like spider nevi, hepatic encephalopathy, or liver tumors.1,2,4,6,8–10,14 In the case described, digital clubbing, a clinical sign suggestive of chronic hypoxemia, was not consistent with a repeatedly observed normal hematocrit (39%) rather than an increased one as expected in view of the hypoxemia. It may be that it was too soon to observe a compensatory polyglobulia, or perhaps hypoxemia should have been more severe to stimulate erytropoyetin production. Orthodeoxia was also observed, a clinical finding that may accompany the hepatopulmonary syndrome.4,12
A mild autism was recognized but liver dysfunction was not detected and a normal liver parenchyma was visualized in the abdominal echography and in the CT. Thus, it is unclear whether or not the autistic features could correspond to an incipient form of hepatic encephalopathy.
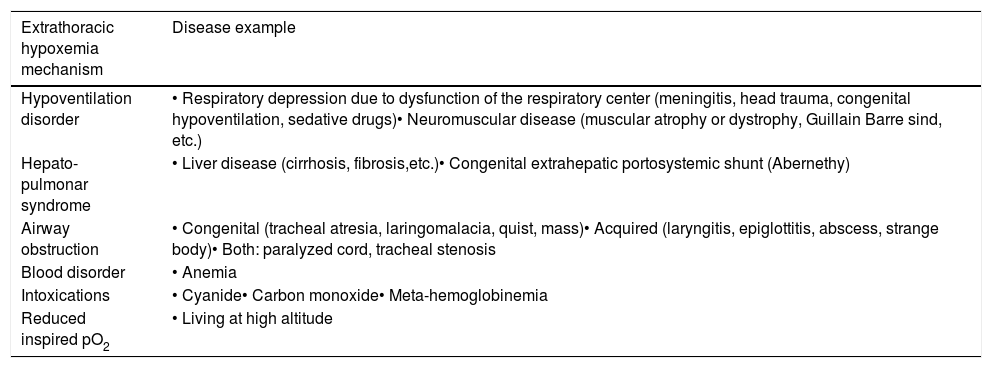
Albeit diagnosis of Abernethy malformation in children remains challenging, once suspected clinically its presence can be easily confirmed by non-invasive imaging techniques such as abdominal echography, CT or Magnetic Resonance Imaging.1,3,5,9 Although this malformation can be a cause of extrathoracic hypoxemia, additional causes are summarized in Table 1.15
Main mechanisms of extrathoracic hypoxemia in pediatrics.
| Extrathoracic hypoxemia mechanism | Disease example |
|---|---|
| Hypoventilation disorder | • Respiratory depression due to dysfunction of the respiratory center (meningitis, head trauma, congenital hypoventilation, sedative drugs)• Neuromuscular disease (muscular atrophy or dystrophy, Guillain Barre sind, etc.) |
| Hepato-pulmonar syndrome | • Liver disease (cirrhosis, fibrosis,etc.)• Congenital extrahepatic portosystemic shunt (Abernethy) |
| Airway obstruction | • Congenital (tracheal atresia, laringomalacia, quist, mass)• Acquired (laryngitis, epiglottitis, abscess, strange body)• Both: paralyzed cord, tracheal stenosis |
| Blood disorder | • Anemia |
| Intoxications | • Cyanide• Carbon monoxide• Meta-hemoglobinemia |
| Reduced inspired pO2 | • Living at high altitude |
According to others and to our experience, optimal management of this congenital shunt requires a multidisciplinary team.8 Medical treatment, a prophylactic or therapeutic shunt closure, and a liver transplant are the main healing options.1–3,5,7–11 In our patient, and given the unfavorable anatomy for interventionism, a surgical closure through laparoscopy was elected. In general, it appears reasonable to perform an early rather than a late shunt closure in order to reduce the development of complications.1,7,9 Shunt closure restores intrahepatic circulation in most patients allowing a clinical improvement of hypoxemia, hepatic encephalopathy, and in some instances of the pulmonary hypertension.1,8,9 Our patient needed six months to recover a normal transcutaneous hemoglobin saturation and one year to experience a notable improvement in his behavior.
The case described contributes to increase the awareness of extrathoracic hypoxemia causes such as Abernethy malformation when facing a chronic hypoxemia in children and once cardiac and primary pulmonary causes have been excluded. A prompt diagnosis and proper management may prevent the development of serious complications.
Financial disclosureThe authors have no financial relationship to disclose.
Conflict of interestThe authors have no conflicts of interest to disclose.