In clinical practice, a diagnosis of pulmonary eosinophilia is suspected in patients with respiratory symptoms (dyspnea, cough or wheezing), migratory pulmonary infiltrates on chest X-ray, and eosinophilia in peripheral blood, or preferably, in the lung.1,2 Hypereosinophilic syndrome (HES) is a rare entity with different forms, one of the most exceptional of which is myeloproliferative HES. We report a case of FIP1L1/PDGFRA-positive myeloproliferative HES diagnosed in a patient with pulmonary eosinophilia.
A 60-year-old man, smoker of 50 pack-years, attended the respiratory medicine clinic with a 2-month history of worsening of his habitual cough and expectoration, and no other symptoms. He had been diagnosed 2 years previously with grade II COPD (forced expiratory volume in 1s [FEV1] 79%), well managed after giving up smoking and receiving treatment with fluticasone/salmeterol 25/250mcg.
No lymphadenopathies were observed on physical examination, and auscultation revealed some rhonchi, and a respiratory rate of 16breaths/min and heart rate of 90beats/min. No other significant findings were noted.
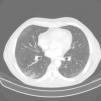
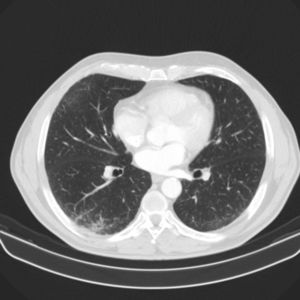
Blood tests showed leukocytosis 12590cells/mm with eosinophils 8560 (61%). Other blood counts, coagulation, and biochemistry results were normal. Lung function tests: FEV1 2610l (87%), forced vital capacity (FVC) 4090l (109%), FEV1/FVC 63.78%. Bronchodilator test and methacholine challenge were negative. Chest X-ray showed radiological signs of COPD. Chest and abdomen CT showed centriacinar and paraseptal emphysema, micronodules in the upper lung lobes, middle lobe and lingula, and thickening of the bladder, with suspected malignancy (Fig. 1). Echocardiogram was normal. Given these findings, the patient was referred to the urology department, where he was diagnosed with transitional cell carcinoma, and treated accordingly. The high number of eosinophils in blood prompted us to undertake a detailed differential diagnosis. Tests for parasites, helminths (Ascaris lumbricoides, Taenia solium, hydatidosis, Toxocara canis, Leishmania) and fungi, and stool culture were all negative. Hepatitis A, B and C and human immunodeficiency virus serologies, and Mycobacterium and Bordetella testing were also negative. In view of these findings, fiberoptic bronchoscopy was performed, and bronchial aspirate, bronchoalveolar lavage (BAL), and transbronchial biopsy (TBB) were obtained. Bronchial aspirate was negative for malignant cells. The BAL cell count was: lymphocytes 6%, polymorphonuclear cells 69%, and eosinophils 20%. Pathology study of TBB reported bronchial mucosa and pulmonary parenchyma with eosinophil infiltration, confirming pulmonary eosinophilia. The immunological study, including antinuclear antibodies, anti-neutrophil cytoplasmic antibodies, and rheumatoid factor, was also negative.
The hematology department was consulted, in view of the patient's persistently high blood and pulmonary eosinophil levels (11.330/μl and 20%, respectively). A bone marrow biopsy was performed which showed marked eosinophilia with predominantly mature cells. Flow cytometry revealed a predominance of CD3. A genetic study was performed using fluorescence in situ hybridization (FISH), a technique which detects and locates a specific DNA sequence on a chromosome after hybridization with a fluorescent molecule. The results were positive for the 4q12 FIP1L1 gene in 20% of cells with normal karyotype. The rest of the genetic analysis was negative. These data and the immunophenotype prompted us to consider a diagnosis of tumor-derived hypereosinophilic syndrome, and allowed us to differentiate between myeloproliferative and lymphoproliferative disease. The patient was thus diagnosed with myeloproliferative hypereosinophilic syndrome with pulmonary involvement, and specific treatment was initiated.
Hypereosinophilic syndrome (HES) is a heterogeneous group of disorders, and causes range from idiopathic disease to malignancy. It mostly affects men aged 20–40 years, with eosinophilia ≥1500cells/mm3 for more than 6 months, and signs of systemic involvement.3 Lung involvement is detected in 40% of cases (cough, pulmonary infiltrates with micronodules on CT, and pleural involvement).4
Our patient presented marked peripheral eosinophilia and a 2-month history of worsening of his respiratory disease (COPD), along with radiological images of lung involvement. The differential diagnosis of this type of pathological process is complex. In patients with pulmonary involvement and eosinophilia, and no evidence of disease in other organs, the first step is to rule out infection, allergy, effects of drugs, and autoimmune diseases.5 With the collaboration of the hematology department, we were able to establish a definitive diagnosis. In this case, eosinophilia in the BAL and TBB prompted us to perform a bone marrow biopsy, which, together with the subsequent genetic assessment, confirmed the diagnosis of FIP1L1/PDGFRA-positive myeloproliferative HES.6,7
There are 2 variants of HES: lymphoproliferative, occurring in 90% of cases, and myeloproliferative, in 10%. This variant is due in most cases to rearrangement of the FIP1L1/PDGFRA gene, although other genetic disorders have also been observed. It is detected using the FISH technique, which can identify chromosomal abnormalities by capturing the fluorescent point on the chromosome to which each FISH probe binds. This mutation causes continuous activation of a tyrosine kinase that leads to the clonal proliferation of eosinophils.8–10
Although HES is a very rare entity that is still relatively unknown, we do not believe that it was associated with the transitional cell carcinoma detected in our patient, although we cannot confidently rule this out.
To conclude, in the evaluation of pulmonary eosinophilia when eosinophil concentrations are very high, the possibility of rare hematological diseases, such as FIP1L1/PDGFRA-positive myeloproliferative HES, must be considered.
Please cite this article as: Sánchez-Jareño M, Yuste Jiménez V, Villasante C, Canales MÁ, Álvarez-Sala R. Varón de 60 años de edad con enfermedad pulmonar obstructiva crónica y eosinofilia. Arch Bronconeumol. 2018;54:394–395.