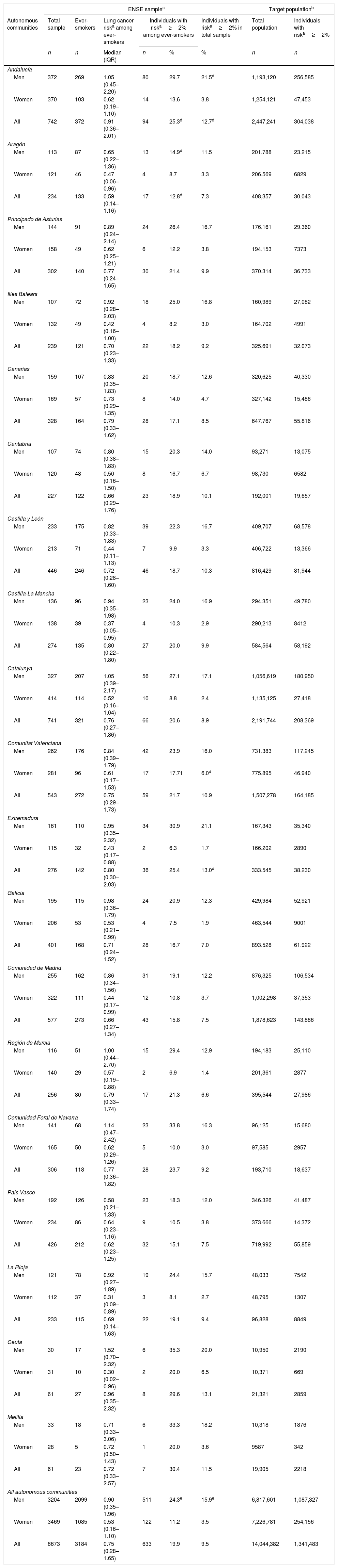
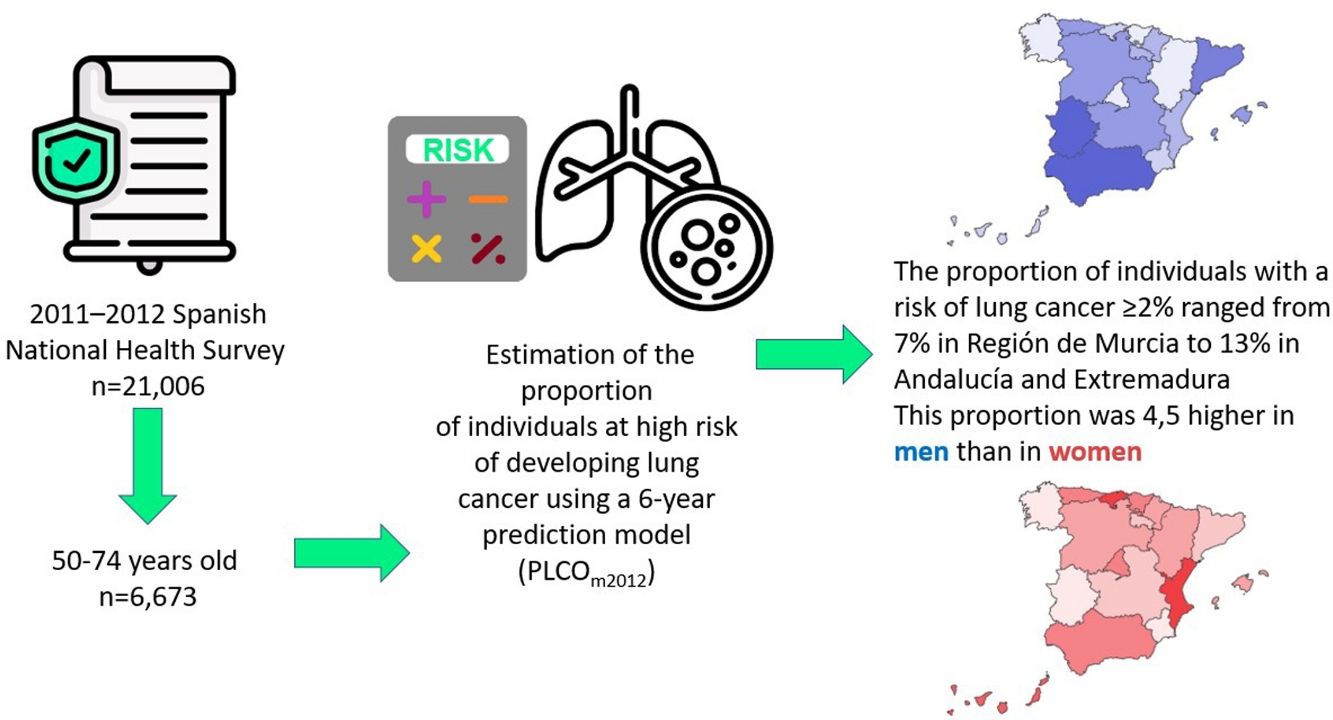
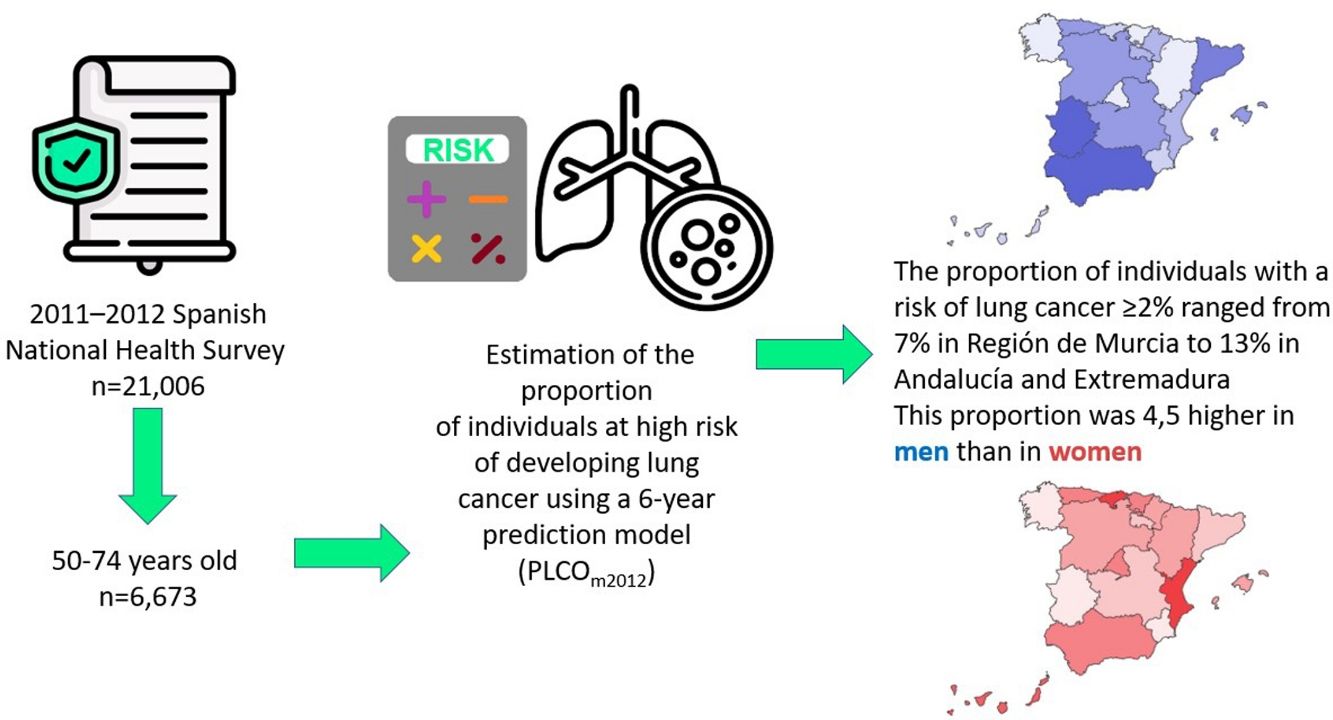
Lung cancer screening with low-dose computed tomography (LDCT) has been proposed as a strategy to reduce lung cancer mortality. Since LDCT has side effects there is a need to carefully select the target population for screening programmes. Because in Spain health competences are transferred to the seventeen Autonomous Communities (ACs), the present paper aims to identify individuals at high risk of developing lung cancer in the different ACs.
MethodsWe used the 2011–2012 data of the Spanish National Interview Health Survey (n=21,006) to estimate the proportion of individuals at high risk of developing lung cancer using a 6-year prediction model (PLCOm2012). This proportion was then extrapolated into absolute figures for the Spanish population, using the population census data of 2018 from the National Institute of Statistics.
ResultsThe proportion of individuals aged 50–74 with a risk of lung cancer ≥2% was 9.5% (15.9% in men, 3.5% in women). This proportion ranged from 6.6% in Región de Murcia to 12.7% in Andalucía and 13.0% in Extremadura. When extrapolated to the Spanish population, it was estimated that a total of 1,341,483 individuals may have a 6-year risk of lung cancer ≥2%.
ConclusionsThe present study is the first one that evaluated the number of individuals at high risk of developing lung cancer in the different Spanish ACs using a prediction model and selecting people with a 6-year risk ≥2%. Further studies should assess the cost and effectiveness associated to the implementation of a lung cancer screening programme to such population.
La detección del cáncer de pulmón con tomografía computarizada de baja dosis se ha propuesto como una estrategia para reducir la mortalidad por dicho cáncer. Como la tomografía computarizada de baja dosis tiene efectos secundarios, es necesario seleccionar cuidadosamente la población objetivo para los programas de cribado. Debido a que en España las competencias de salud están transferidas a las 17 comunidades autónomas, el presente trabajo tiene como objetivo identificar a las personas con alto riesgo de desarrollar cáncer de pulmón en las diferentes comunidades autónomas.
MétodosUtilizamos los datos de 2011-2012 de la Encuesta Nacional de Salud de España (n=21.006) para estimar la proporción de individuos con alto riesgo de desarrollar cáncer de pulmón utilizando un modelo de predicción a 6 años (PLCOm2012). Esta proporción se extrapoló en cifras absolutas para la población española, utilizando los datos del censo de población de 2018 del Instituto Nacional de Estadística.
ResultadosLa proporción de individuos de 50 a 74 años con riesgo de cáncer de pulmón≥2% fue del 9,5% (15,9% en hombres, 3,5% en mujeres). Esta proporción osciló entre el 6,6% en la Región de Murcia, el 12,7% en Andalucía y el 13,0% en Extremadura. Cuando se extrapoló a la población española, se estimó que un total de 1.341.483 individuos podrían tener un riesgo de cáncer de pulmón a los 6 años≥2%.
ConclusionesEl presente estudio es el primero que evaluó el número de individuos con alto riesgo de desarrollar cáncer de pulmón en las diferentes comunidades autónomas españolas utilizando un modelo predictivo y seleccionando personas con un riesgo a los 6 años≥2%. Se deberían realizar estudios adicionales para evaluar el coste y la efectividad asociados a la implementación de un programa de cribado de cáncer de pulmón para dicha población.
Lung cancer is the most common cause of cancer-related death in Europe1 with an age-standardised 5-year survival of 13% for adult patients with cancer diagnosed in 2000–2007.2 In Spain, according to the Spanish Network of Cancer Registries (REDECAN), lung cancer will be in 2020 the most common cancer with an expected incidence rate (adjusted on the actual European population) of 64.1 per 100,000 individuals.3 For the period 2008–2013, the age-standardised 5-year relative survival was 13% and 18% for Spanish men and women respectively.4
Tobacco smoking is the main risk factor for developing lung cancer, with up to 90% of lung cancer attributed to smoking.5 Although smoking cessation has been identified as the most cost-effective strategy to prevent lung cancer,6 it may not be achieved in all current smokers; also, former smokers may still be at high risk of developing lung cancer. Thus, other strategies, alone or in combination, can be considered to better reduce lung cancer mortality.
Lung cancer screening with low-dose computed tomography (LDCT) has been proposed as an early-detection strategy to reduce lung cancer mortality.7 A meta-analysis, that combined the results of most randomized trials assessing the effects of lung cancer screening with LDCT, concluded that it might significantly reduce lung cancer mortality by 17%.8 These results support the European Union position statement published in 2017 that recommended the implementation of lung cancer screening with LDCT in Europe arguing that it can save lives.9 However, because lung cancer screening with LDCT is not exempt from side effects, this statement also recommended the adoption of specific actions, such as use of a risk stratification approach, to ensure a successful implementation lung cancer screening with LDCT. These side effects, that include physical and psychological harms due to overdiagnosis, surgery for benign lesions or radiation exposure,10 stress the need to carefully select the target population for lung cancer screening programmes and the importance of incorporating tobacco cessation practices in all settings.11
Recent studies have shown that a selection of candidates for lung cancer screening based on high-quality risk prediction models is superior to a selection based on criteria such as age and pack-years alone as it leads to fewer individuals being screened, more cancers being detected, and fewer false positives.12,13
In a previous paper, we compared different strategies to identify the proportion of the Spanish population at high risk of developing lung cancer, susceptible to be included in a lung cancer screening programme.14 Because in Spain health competences are transferred to the seventeen Autonomous Communities (ACs), and each autonomous region has competence to organize the prevision of preventive services, the present paper describes the proportions and absolute numbers of individuals at high risk of developing lung cancer in the different Spanish ACs.
MethodsStudy design and subjectsThe present study uses data from the 2011 to 2012 Spanish National Health Survey (Encuesta Nacional de Salud de España, ENSE), a cross-sectional study on a representative sample of the non-institutionalized Spanish population aged ≥15 years old. The ENSE, conducted every five years, gathers health-related information at national level. Detailed information on the ENSE methodology is available on the website of the Spanish Ministry of Health (www.msssi.gob.es/en/estadEstudios/estadisticas/encuestaNacional/ense.htm).
Briefly, survey participants were selected by means of probabilistic multistage sampling in order to obtain representative data at regional and national level. The sampling method consisted of a multistage cluster, where primary units were census tracts, secondary units were households and the tertiary units (individuals) were selected from the description of household members at the time of the interview. A sex and age-stratified sampling scheme was used for this survey.
For the present analysis, no consent statement from participants was necessary, as all microdata are anonymised and openly available on the aforementioned website. However, the overall project received the approval of the Clinical Research Ethics Committee of the Bellvitge University Hospital (ref PR249/16).
In 2011–2012, the ENSE data included information on 21,006 individuals ≥15 years old. To assess the risk of developing lung cancer in Spain, by ACs and sex, ENSE participants aged 50–74 (n=7597) were selected. This age range was previously used in the Dutch-Belgian randomized lung cancer screening trial (NELSON), the second largest randomized controlled trial in demonstrating a reduction in lung cancer mortality after lung cancer screening with LDCT.15
Variables and analysisThe risk of lung cancer was estimated using the model developed in the context of the prostate, lung, colorectal and ovarian screening trial (PLCO trial).16 The validated 6-year prediction model for ever-smokers (i.e. current and former smokers) developed by Tammemägi et al. (PLCOm2012) includes age, race/ethnicity, education, body mass index, personal history of cancer, family history of lung cancer, chronic obstructive pulmonary disease (COPD), smoking status, tobacco consumption, smoking duration and time since quitting for former smokers.17 We used this prediction model, but we did not include the family history of lung cancer and ethnicity variables, as this information was not available in the ENSE survey, therefore, we assumed there was no risk due to family history of lung cancer and that all population was Caucasian. For the education variable, we used the socioeconomic status of the head of household, that includes the following six categories: professions associated to postgraduate university degrees; professions associated to graduate university degrees and qualified technicians; administrative employees and professionals, personal service and self-employed workers, and supervisors of manual workers; skilled and semi-skilled manual workers; and unskilled workers.18
In the present study, ever-smokers with an estimated risk of developing lung cancer ≥2% were considered at high risk. This threshold was obtained in a previous analysis, also based on the 2011–2012 ENSE survey, comparing different strategies to identify the proportion of the Spanish population at high risk of developing lung cancer susceptible to be included in a screening programme.14 This analysis, that started from the high-risk criteria from the National Lung Screening Trial (ever-smokers aged 55–74 years old having smoked ≥30 pack-years and with ≤15 years since cessation for quitters), showed that 2.5% of the Spanish population fulfilling the NLST criteria had a 6-year lung cancer risk ≥2.0%. The 2.0% threshold had previously been used in a study aimed to validate the performance of PLCOm2012 in predicting lung cancer outcomes in a cohort of Australian smokers that showed that it performed better than the NLST criteria.19
The present paper shows the median and interquartile range (IQR: percentile 25–percentile 75) of the 6-year individual risk of developing lung cancer of ever-smokers calculated with the PLCOm2012 model by sex and AC. It also indicates the number of ever-smokers having an individual risk ≥2.0% and the proportion they represent both in the ever-smoker and in the total populations. The proportions observed in the different ACs were then compared to the proportion observed at national level in order to identify possible geographic variations.
The proportions of participants at high risk of developing lung cancer of the different ACs obtained from the ENSE sample were then extrapolated into absolute figures for the Spanish population, using the latest available population census data of 2018 from the National Institute of Statistics (www.ine.es).
Due to missing values for at least one of the variables involved in the calculation of the PLCOm2012 model, 924 subjects were excluded from the present analysis that was therefore performed on 6673 subjects.
ResultsAmong the 6673 ENSE subjects, aged 50–74 years involved in this analysis, 3184 (2099 men and 1085 women) were ever-smokers (Table 1). Their median 6-year risk of developing lung cancer was 0.90 (IQR 0.35–1.96) for men and 0.53 (IQR 0.16–1.10) for women.
| ENSE samplec | Target populationb | |||||||
|---|---|---|---|---|---|---|---|---|
| Autonomous communities | Total sample | Ever-smokers | Lung cancer riska among ever-smokers | Individuals with riska≥2% among ever-smokers | Individuals with riska≥2% in total sample | Total population | Individuals with riska≥2% | |
| n | n | Median (IQR) | n | % | % | n | n | |
| Andalucía | ||||||||
| Men | 372 | 269 | 1.05 (0.45–2.20) | 80 | 29.7 | 21.5d | 1,193,120 | 256,585 |
| Women | 370 | 103 | 0.62 (0.19–1.10) | 14 | 13.6 | 3.8 | 1,254,121 | 47,453 |
| All | 742 | 372 | 0.91 (0.36–2.01) | 94 | 25.3d | 12.7d | 2,447,241 | 304,038 |
| Aragón | ||||||||
| Men | 113 | 87 | 0.65 (0.22–1.36) | 13 | 14.9d | 11.5 | 201,788 | 23,215 |
| Women | 121 | 46 | 0.47 (0.06–0.96) | 4 | 8.7 | 3.3 | 206,569 | 6829 |
| All | 234 | 133 | 0.59 (0.14–1.16) | 17 | 12.8d | 7.3 | 408,357 | 30,043 |
| Principado de Asturias | ||||||||
| Men | 144 | 91 | 0.89 (0.24–2.14) | 24 | 26.4 | 16.7 | 176,161 | 29,360 |
| Women | 158 | 49 | 0.62 (0.25–1.21) | 6 | 12.2 | 3.8 | 194,153 | 7373 |
| All | 302 | 140 | 0.77 (0.24–1.65) | 30 | 21.4 | 9.9 | 370,314 | 36,733 |
| Illes Balears | ||||||||
| Men | 107 | 72 | 0.92 (0.28–2.03) | 18 | 25.0 | 16.8 | 160,989 | 27,082 |
| Women | 132 | 49 | 0.42 (0.16–1.00) | 4 | 8.2 | 3.0 | 164,702 | 4991 |
| All | 239 | 121 | 0.70 (0.23–1.33) | 22 | 18.2 | 9.2 | 325,691 | 32,073 |
| Canarias | ||||||||
| Men | 159 | 107 | 0.83 (0.35–1.83) | 20 | 18.7 | 12.6 | 320,625 | 40,330 |
| Women | 169 | 57 | 0.73 (0.29–1.35) | 8 | 14.0 | 4.7 | 327,142 | 15,486 |
| All | 328 | 164 | 0.79 (0.33–1.62) | 28 | 17.1 | 8.5 | 647,767 | 55,816 |
| Cantabria | ||||||||
| Men | 107 | 74 | 0.80 (0.38–1.83) | 15 | 20.3 | 14.0 | 93,271 | 13,075 |
| Women | 120 | 48 | 0.50 (0.16–1.50) | 8 | 16.7 | 6.7 | 98,730 | 6582 |
| All | 227 | 122 | 0.66 (0.29–1.76) | 23 | 18.9 | 10.1 | 192,001 | 19,657 |
| Castilla y León | ||||||||
| Men | 233 | 175 | 0.82 (0.33–1.83) | 39 | 22.3 | 16.7 | 409,707 | 68,578 |
| Women | 213 | 71 | 0.44 (0.11–1.13) | 7 | 9.9 | 3.3 | 406,722 | 13,366 |
| All | 446 | 246 | 0.72 (0.28–1.60) | 46 | 18.7 | 10.3 | 816,429 | 81,944 |
| Castilla-La Mancha | ||||||||
| Men | 136 | 96 | 0.94 (0.35–1.98) | 23 | 24.0 | 16.9 | 294,351 | 49,780 |
| Women | 138 | 39 | 0.37 (0.05–0.95) | 4 | 10.3 | 2.9 | 290,213 | 8412 |
| All | 274 | 135 | 0.80 (0.22–1.80) | 27 | 20.0 | 9.9 | 584,564 | 58,192 |
| Catalunya | ||||||||
| Men | 327 | 207 | 1.05 (0.39–2.17) | 56 | 27.1 | 17.1 | 1,056,619 | 180,950 |
| Women | 414 | 114 | 0.52 (0.16–1.04) | 10 | 8.8 | 2.4 | 1,135,125 | 27,418 |
| All | 741 | 321 | 0.76 (0.27–1.86) | 66 | 20.6 | 8.9 | 2,191,744 | 208,369 |
| Comunitat Valenciana | ||||||||
| Men | 262 | 176 | 0.84 (0.39–1.79) | 42 | 23.9 | 16.0 | 731,383 | 117,245 |
| Women | 281 | 96 | 0.61 (0.17–1.53) | 17 | 17.71 | 6.0d | 775,895 | 46,940 |
| All | 543 | 272 | 0.75 (0.29–1.73) | 59 | 21.7 | 10.9 | 1,507,278 | 164,185 |
| Extremadura | ||||||||
| Men | 161 | 110 | 0.95 (0.35–2.32) | 34 | 30.9 | 21.1 | 167,343 | 35,340 |
| Women | 115 | 32 | 0.43 (0.17–0.88) | 2 | 6.3 | 1.7 | 166,202 | 2890 |
| All | 276 | 142 | 0.80 (0.30–2.03) | 36 | 25.4 | 13.0d | 333,545 | 38,230 |
| Galicia | ||||||||
| Men | 195 | 115 | 0.98 (0.36–1.79) | 24 | 20.9 | 12.3 | 429,984 | 52,921 |
| Women | 206 | 53 | 0.53 (0.21–0.99) | 4 | 7.5 | 1.9 | 463,544 | 9001 |
| All | 401 | 168 | 0.71 (0.24–1.52) | 28 | 16.7 | 7.0 | 893,528 | 61,922 |
| Comunidad de Madrid | ||||||||
| Men | 255 | 162 | 0.86 (0.34–1.56) | 31 | 19.1 | 12.2 | 876,325 | 106,534 |
| Women | 322 | 111 | 0.44 (0.17–0.99) | 12 | 10.8 | 3.7 | 1,002,298 | 37,353 |
| All | 577 | 273 | 0.66 (0.27–1.34) | 43 | 15.8 | 7.5 | 1,878,623 | 143,886 |
| Región de Murcia | ||||||||
| Men | 116 | 51 | 1.00 (0.44–2.70) | 15 | 29.4 | 12.9 | 194,183 | 25,110 |
| Women | 140 | 29 | 0.57 (0.19–0.88) | 2 | 6.9 | 1.4 | 201,361 | 2877 |
| All | 256 | 80 | 0.79 (0.33–1.74) | 17 | 21.3 | 6.6 | 395,544 | 27,986 |
| Comunidad Foral de Navarra | ||||||||
| Men | 141 | 68 | 1.14 (0.47–2.42) | 23 | 33.8 | 16.3 | 96,125 | 15,680 |
| Women | 165 | 50 | 0.62 (0.29–1.26) | 5 | 10.0 | 3.0 | 97,585 | 2957 |
| All | 306 | 118 | 0.77 (0.36–1.82) | 28 | 23.7 | 9.2 | 193,710 | 18,637 |
| Pais Vasco | ||||||||
| Men | 192 | 126 | 0.58 (0.21–1.33) | 23 | 18.3 | 12.0 | 346,326 | 41,487 |
| Women | 234 | 86 | 0.64 (0.23–1.16) | 9 | 10.5 | 3.8 | 373,666 | 14,372 |
| All | 426 | 212 | 0.62 (0.23–1.25) | 32 | 15.1 | 7.5 | 719,992 | 55,859 |
| La Rioja | ||||||||
| Men | 121 | 78 | 0.92 (0.27–1.89) | 19 | 24.4 | 15.7 | 48,033 | 7542 |
| Women | 112 | 37 | 0.31 (0.09–0.89) | 3 | 8.1 | 2.7 | 48,795 | 1307 |
| All | 233 | 115 | 0.69 (0.14–1.63) | 22 | 19.1 | 9.4 | 96,828 | 8849 |
| Ceuta | ||||||||
| Men | 30 | 17 | 1.52 (0.70–2.32) | 6 | 35.3 | 20.0 | 10,950 | 2190 |
| Women | 31 | 10 | 0.30 (0.02–0.96) | 2 | 20.0 | 6.5 | 10,371 | 669 |
| All | 61 | 27 | 0.96 (0.35–2.32) | 8 | 29.6 | 13.1 | 21,321 | 2859 |
| Melilla | ||||||||
| Men | 33 | 18 | 0.71 (0.33–3.06) | 6 | 33.3 | 18.2 | 10,318 | 1876 |
| Women | 28 | 5 | 0.72 (0.50–1.43) | 1 | 20.0 | 3.6 | 9587 | 342 |
| All | 61 | 23 | 0.72 (0.33–2.57) | 7 | 30.4 | 11.5 | 19,905 | 2218 |
| All autonomous communities | ||||||||
| Men | 3204 | 2099 | 0.90 (0.35–1.96) | 511 | 24.3e | 15.9e | 6,817,601 | 1,087,327 |
| Women | 3469 | 1085 | 0.53 (0.16–1.10) | 122 | 11.2 | 3.5 | 7,226,781 | 254,156 |
| All | 6673 | 3184 | 0.75 (0.28–1.65) | 633 | 19.9 | 9.5 | 14,044,382 | 1,341,483 |
IQR, interquartile range.
Based on the PLCOm2012 model for ever-smokers. The model included the following variables: age, socioeconomic status, body mass index, COPD, personal history of cancer, smoking status, tobacco consumption, smoking duration and years of abstinence.
Target population using Spanish population census data of 2018 (www.ine.es) restricted to individuals 50–74 years old.
Overall, the proportion of 50–74 years old ENSE participants with a risk of lung cancer ≥2% significantly varied across sex. Among men, 511 ever-smokers showed a risk of developing lung cancer ≥2%; they represent almost a quarter (24.3%) of male ever-smokers and 15.9% of the male ENSE sample. In women, 122 ever-smokers had a risk of developing lung cancer ≥2%, which represents 11.2% of ever-smokers and 3.5% of the whole female ENSE sample.
In the ENSE sample, the ACs with the highest proportion of individuals at risk of lung cancer ≥2% were Ceuta (13.1%), Extremadura (13.0%), Andalucía (12.7%) and Melilla (11.5%), while the ACs with the lowest proportions of ever-smokers at risk ≥2% were Región de Murcia (6.6%), Galicia (7.0%), Aragón (7.3%), Comunidad de Madrid (7.5%) and País Vasco (7.5%). The proportions of individuals with a risk of lung cancer ≥2% observed in Andalucía and Extremadura were significantly above the proportion observed at national level (9.5%; p-values of 0.006 and 0.050, respectively).
We extrapolated these proportions into absolute figures for the Spanish population aged 50–74 years old and obtained that 1,341,483 individuals (1,087,327 men and 254,156 women) may have a risk of developing lung cancer ≥2% and might therefore benefit from lung cancer screening. The total number of individuals having a high-risk of lung cancer ranged from 8849 in La Rioja to 304,038 in Andalucía. While 17% of the Spanish population live in Andalucía, the number of individuals having a high-risk of lung cancer in this AC represented 23% of the total number of individuals having a high-risk of lung cancer.
DiscussionThe present study showed that 9.5% of the adult Spanish population had a 6-year risk of developing lung cancer, calculated using the model developed in the context of the PLCO trial, above the threshold of 2%. The proportion of subjects at high risk was significantly higher in men (15.9%) than in women (3.5%) and represented a total of more than 1,3 million people. However, the proportions observed in the different ACs were not very different from those observed at national level.
To our knowledge, only two papers have previously reflected on the use of LDCT for lung cancer screening in Spain.20,21 Ruano-Ravina and colleagues provided an estimation of the number of men and women who fulfilled lung cancer screening criteria in the different Spanish ACs.20 They estimated that a total of 1,714,683 individuals fulfilled lung cancer screening criteria. Although based on the same data (ENSE 2011–2012) this figure is above ours. This difference is explained by the age range selected in both studies (55–80 years old in their analysis and 50–74 years old in ours) and by the criteria used to define individuals at high-risk of developing lung cancer. While they used the NLST criteria (based on age and smoking history), we used a predictive model and selected individuals with a risk above the threshold of 2%; a selection strategy that previously showed to be more restrictive than the NLST criteria.14 The second paper, set up recommendations about the implementation of LDCT for lung cancer screening, but did not provide any estimation of the target population.21 The authors mentioned that, despite the accumulated scientific evidence on the benefits of lung cancer screening, some doubts still persist regarding the feasibility of its large-scale implementation mainly due to the uncertainty regarding the selection of candidates, the number and frequency of the scans and the management of findings. For this reason they recommend the design of pilot studies to analyze the benefits and risks of lung cancer screening in the Spanish environment.
In Spain, the Pamplona International Early Lung Cancer Detection Program (P-IELCAP) is the longest ongoing lung cancer screening programme.22 It has been ongoing in a private centre since 2000 as part of a single arm trial called I-ELCAP that started to screen with LDCT asymptomatic persons at risk for lung cancer in 1993.23 In 2015, Sanchez-Salcedo and colleagues published the first results of the P-IELCAP based on 2,989 men and women, aged 40 years and older, current or former smokers with a smoking history of ≥10 pack-years who had been screened between 2000 and 2014.22 While the authors concluded that the Spanish experience of lung cancer screening showed findings comparable with those observed in the rest of Europe and confirmed the feasibility and efficacy of lung cancer screening using LDCT, they also mentioned that the P-IELCAP performed in a private centre may not be entirely applicable to public institutions. This last point was also discussed in the reflection paper of Ruano-Ravina and colleagues, who wondered whether the Spanish public health system would be able to (i) identify the target population for lung cancer screening taking into account the information available in medical records (smoking history is not always registered); (ii) face the costs of a LDCT screening programme (tomographs and health professionals involved in screening); (iii) guarantee adequate participation of the target population and (iv) allocate extra medical resources to handle overdiagnosis and surgery for benign lesions.20 More recently, Ruano-Ravina et al. have indicated that the available evidence does not support the idea that the benefits of lung cancer screening with LDCT are greater than the harms, and recommended the funding of smoking cessation programmes in order to effectively reduce lung cancer burden.24 While funding smoking cessation programmes is necessary to reduce lung cancer incidence and improve cancer treatments,25 it is important to keep in mind that all current smokers may not be able to quit smoking and that some former smokers will still be at risk of developing lung cancer and may therefore benefit from lung cancer screening. Previous studies have evaluated the effect of introducing smoking cessation interventions in the context of lung cancer screening and showed that participation in a lung screening trial may promote cessation.26
Although some experts still actually think that the risk–benefit balance of lung cancer screening with LDCT is questionable, and do not recommend its implementation,24 expert panels have invited European countries to actively start a widespread implementation of lung cancer screening.9 Since screening of lung cancer with LDCT may produce physical and psychological harms, mainly due to overdiagnosis, surgery for benign lesions or radiation exposure,10 this prevention strategy needs to be offered to subjects at high risk of developing lung cancer in order to achieve the highest benefit-to-harm ratio. To achieve this goal, it is essential to use a risk assessment tool such as the PLCOm2012 that incorporates sociodemographic and health-related factors. This model previously showed to be superior to the NLST eligibility criteria (based on age and smoking history) and demonstrated excellent predictive performance in a large Australian cohort of 95,822 smokers, as well as higher positive predictive value and sensitivity, with minimal loss of specificity.19
To actively start the implementation of lung cancer screening, as recommended by European stakeholders, there is a crucial need to get precise information on the size of the target population, based on prediction models that include the most relevant risk factors for lung cancer, for which reliable data can be systematically obtained. Our analysis showed that the total number of high risk people does not only depend on the population size but on the prevalence of smoking. Indeed, while an AC such as Andalucia included 17% of the Spanish population, the number of individuals at high-risk of lung cancer in this AC represented 23% of the total number of individual at high-risk of lung cancer. Using the results described in the present paper, the health authorities of the different ACs willing to set-up a lung cancer screening programme in their region will be able to estimate the cost of such programme and/or design feasibility and pilot studies. Taking into account the actual economic constraints on the Spanish healthcare system, before implementing new prevention strategies, it is important to precisely evaluate the costs and the effectiveness of these strategies.
The present paper has limitations that need to be acknowledged. While the most recent ENSE data were published in 2017, we used data from 2011 to calculate to the proportion of individuals at high risk of lung cancer, as the latest survey did not include information on smoking intensity in ex-smokers and in current non-cigarette smokers (pipe or cigar). When we compared both sets of data, we observed similar proportions of current cigarette smokers (20% and 19% in 2011 and 2017, respectively) as well as comparable proportions of current cigarette smokers having a 6-year risk of developing lung cancer (7% and 8% in 2011 and 2017, respectively). For this reason, we think that if the analysis had been performed on the most recent ENSE data available, the results obtained would not significantly differ from those showed in the present paper. While the PLCOm2012 risk model includes family history of lung cancer and race/ethnicity as additional factors in the identification of ever-smokers at highest risk of developing lung cancer, we were not able to include these variables as this information was not gathered by the ENSE survey. Nevertheless, ethnicity is not such a relevant variable in Spain, as it can be in other countries, because the proportion of Caucasian is 93–95% and the remaining part of the population includes different ethnicities, Hispanic being the main one.27 For this reason, we think that assuming that our population was Caucasian may have produced a slight over estimation of the risk of lung cancer while assuming that no one had a family history of lung cancer may have resulted in a slight under estimation of this risk, taking into account the coefficients of the variables Hispanic ethnicity and history of lung cancer in Tammemägi's model. Introducing environmental risk factors such as radon or asbestos exposure in prediction models would also be interesting5; however, these factors are rarely systematically reported in medical files.
The main strengths of the present study relied on the use of a prediction model to assess the risk of developing lung cancer in 6-year time and the presentation of national and regional figures in a country in which autonomous regions have their own healthcare services responsible for the health centres, services and facilities of the regions (the Central Government retains healthcare management in the cities of Ceuta and Melilla).
ConclusionsThe present study is the first one that evaluated the number of individuals at high risk of developing lung cancer in the different Spanish ACs using a predictive model to calculate this risk and selecting people with a 6-year risk above the threshold of 2%. This study showed that 9.5% of the overall population (more than 1.3 million of ever-smokers) had a high risk of developing lung cancer, with no major differences observed across ACs. Further studies should assess the cost and effectiveness associated to the implementation of a lung cancer screening programme to such population.
AuthorshipMG and CV conceived the study. NT performed the analysis and prepared the first draft. All authors contributed to the successive reviews of the manuscript and approved its final version.
Funding statementThis study has been funded by the Asociación Española Contra el Cáncer de Barcelona in the Call for proposals of Research in Oncology of 2016 (grant PROYBAR16909FU). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The Tobacco Control Research Group is partly supported by the Ministry of Business and Knowledge from the Government of Catalonia [2017SGR319] and by Instituto de Salud Carlos III, Government of Spain (CIBERES CB19/06/00004). EF was supported by the Instituto de Salud Carlos III, Government of Spain, co-funded by the European Regional Development Fund (FEDER) (INT16/00211 and INT17/00103). We thank CERCA Programme Generalitat de Catalunya for the institutional support.
Conflict of interestsThe authors declare no competing interests.
The members of the Lung Cancer Prevention (LUCAPREV) research group are (in alphabetical order): Catalan Institute of Oncology, L’Hospitalet de Llobregat, Barcelona: Llúcia Benito, Gemma Binefa, Mireia Díaz, Esteve Fernández, Marcela Fu, Montserrat Garcia, Ernest Nadal, Albert Santiago, Noémie Travier, Carmen Vidal. Catalan Cancer Strategy, Department of Health, Generalitat de Catalunya, Barcelona: Julieta Corral. Costa de Ponent Primary Care Directorate, Catalan Institute of Health, L’Hospitalet de Llobregat, Barcelona: Amparo Romaguera. Institut de Recerca Biomèdica de Lleida, University of Lleida, Lleida: Montserrat Rué. Faculty of Economics and Social Sciences, Universitat Internacional de Catalunya, Barcelona: Marta Trapero-Bertran. Primary Care University Research Institute (IDIAP, Jordi Gol), Barcelona, Spain: Carlos Martín-Cantera. University Rovira i Virgili, Reus: Misericòrdia Carles.