Thoracic ultrasound (TU) has rapidly gained popularity over the past 10 years. This is in part because ultrasound equipment is available in many settings, more training programmes are educating trainees in this technique, and ultrasound can be done rapidly without exposure to radiation.
The aim of this review is to present the most interesting and innovative aspects of the use of TU in the study of thoracic diseases.
In pleural diseases, TU has been a real revolution. It helps to differentiate between different types of pleural effusions, guides the performance of pleural biopsies when necessary and is more cost-effective under these conditions, and assists in the decision to remove thoracic drainage after talc pleurodesis.
With the advent of COVID19, the use of TU has increased for the study of lung involvement. Nowadays it helps in the diagnosis of pneumonias, tumours and interstitial diseases, and its use is becoming more and more widespread in the Pneumology ward.
In recent years, TU guided biopsies have been shown to be highly cost-effective, with other advantages such as the absence of radiation and the possibility of being performed at bedside. The use of contrast in ultrasound to increase the cost-effectiveness of these biopsies is very promising.
In the study of the mediastinum and peripheral pulmonary nodules, the introduction of echobronchoscopy has brought about a radical change. It is a fully established technique in the study of lung cancer patients. The introduction of elastography may help to further improve its cost-effectiveness.
In critically-ill patients, diaphragmatic ultrasound helps in the assessment of withdrawal of mechanical ventilation, and is now an indispensable tool in the management of these patients. In neuromuscular patients, ultrasound is a good predictor of impaired lung function. Currently, in Neuromuscular Disease Units, TU is an indispensable tool. Ultrasound study of the intercostal musculature is also effective in the study of respiratory function, and is widely used in Respiratory Rehabilitation.
In Intermediate Care Units, thoracic ultrasound is indispensable for patient management. In these units there are ultrasound protocols for the management of patients with acute dyspnoea that have proven to be very effective.
Thoracic ultrasound (TU) has undergone a genuine revolution in recent years with the appearance of multiple applications and recommendations in different clinical guidelines,1–3 and has developed as a key diagnostic tool in the hands of pulmonologists. The aim of the following review is to present the most interesting and innovative aspects of the use of TU in the study of thoracic diseases.
PleuraTU can identify the presence of pleural effusion (PE) and whether there are fibrin septa, classifying effusions as simple, complex non-septated or complex septated, which helps practitioners to make immediate therapeutic decisions. To explore the thoracic cavity in depth, it is advisable to use microconvex probes, as they facilitate exploration of the intercostal space with good spatial resolution.4
Increased echogenicity, the presence of PE compartmentalization and pleural thickening are present in exudative PE and very often in empyema,5,6 while homogeneous echogenic PE is typical of haemothorax. Malignant PEs, unlike those of benign origin, often present increased thickness of the visceral and/or parietal pleura, in addition to the presence of nodules, which are the best predictors of malignancy.7 In this field, several studies have evaluated the echogenicity of pleural fluid as measured by the pixel density of the ultrasound image, which can differentiate the type of PE, and have found that pixel density is higher in exudative type PE when compared to transudates and correlates with LDH and proteins present in the pleural fluid.8
With respect to pleural thickness, a recent study using B-mode ultrasound showed that selecting 15mm as the cut-off value produced a sensitivity of 78.6%, a specificity of 74.1% and an area under the curve of 0.714 in the diagnosis of malignant pleural disease.9 Although benign pleural diseases may also present with pleural thickening, it is usually uniform as opposed to malignant thickening, perhaps because the thickening is mainly of an exudative fibrous nature or because of hyperplasia of the granulation tissue. Malignant pleural thickening caused by tumours, however, is accompanied by erosion, destruction, or local growth, resulting in the presence of nodules and/or masses.
In fact, recent TU findings in suspected pleural pathology may change the standard diagnostic procedure.10 Thus, TE-guided closed pleural biopsy has been shown to be as cost-effective as thoracoscopic pleural biopsy in patients with exudative PE, and it is associated with a shorter procedure and hospital stay, and fewer complications.11
Non-expandable lung occurs because of chronic pleural processes, including pleural effusions of different etiologies, bronchial obstructions, or atelectasis. The importance of its diagnosis lies in the fact that patients often undergo procedures such as thoracentesis or thoracoscopy, and the presence of non-expandable lung can lead to chest discomfort and even pneumothorax. These complications can be avoided if lung expansion is assessed by pleural manometry12 or TU.13,14 Comparing the two techniques, manometry was found to be more effective than TU as a method of diagnosing lung expansion in patients undergoing pleurodesis.15
Another area which has received little study is when to remove the thoracic drain after talc pleurodesis. The British Thoracic Society criteria, based on the volume drained and chest radiographic findings, are usually followed.16 When these criteria were compared with TU, the latter was shown to allow earlier and equally effective removal of the drain at three months. In patients who underwent daily TU to detect the presence of lung slippage, nine chest regions were studied to obtain a score depending on the presence of pleural slippage: present (1 point), questionable (2 points) or absent (3 points), with the lowest possible resulting score of 9 (slippage preserved) and the highest possible score of 27 (total absence of slippage), and with the drainage tube being removed if the score was above 20.17
DiaphragmThe usefulness of diaphragmatic ultrasound in critically-ill patients in the assessment of withdrawal of mechanical ventilation is now widely established.18–20 In neuromuscular patients, ultrasound appears to be a good predictor of impaired pulmonary function and hypoventilation.21,22
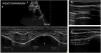
For this, the main diaphragmatic measurements are excursion (mobility), thickness and shortening fraction. Mobility (diaphragmatic excursion) assesses the ability to generate volume changes in the rib cage. A low frequency probe (convex 3.5–5MHz) is used, initially in B-mode via a subcostal approach at the level of the clavicular midline. After locating the posterior third of the diaphragmatic dome, the probe is switched to M-mode to identify the curve corresponding to the respiratory cycle (Fig. 1a). The upper part of the curve corresponds to the position of the diaphragm at the moment of maximum inspiration and the lower part to the position of the diaphragm at the moment of maximum expiration when performing the manoeuvre. To take the measurement, a vertical line must be drawn from the highest to the lowest point of the curve, and it must be performed in three breathing manoeuvres, with the proposed result being the average of the three measurements. There are no standardized reference values, but normal values have been proposed for breathing at rest (tidal volume), inspiration and deep expiration (vital capacity) and sniffing.23 In cases of diaphragmatic paralysis, the movement will be paradoxical and, in cases of paresis, decreased.24 In addition to this, diaphragmatic thickness allows us to assess diaphragmatic muscle mass. A high frequency probe (linear probe 7–10MHz) is used in B-mode through a transthoracic approach in the axillary midline, between the 7th to 10th intercostal spaces. The “apposition zone” is located where the diaphragm inserts into the costal wall. The diaphragm is identified as a hypoechogenic strip bordered by two hyperechogenic lines (pleura and peritoneum). Thickness measurements are taken at the end of unforced expiration (Fig. 1b and c) and there is some variability between normality figures.25–27 In general, the lower limit of normality is usually 0.15cm, when measured in the supine position.25 Finally, the shortening fraction can be assessed. During inspiration, the muscle fibres contract, increasing the thickness of the diaphragm. This increase can be calculated as a percentage and represents an indirect approximation of diaphragmatic contractile capacity. A shortening fraction greater than 20% is considered normal (Fig. 2).28
(a) Ultrasound assessment of diaphragmatic mobility in B-mode and M-mode. Calculation of diaphragmatic excursion: line from the end of expiration to the maximum of the inspiratory curve. (b) Thickness measurement with a linear probe at the end of inspiration and (c) at the end of expiration.
Further studies are needed to standardize these measurements and establish protocols that include ultrasound in the assessment of non-invasive mechanical ventilation and in neuromuscular patients.29
LungAll findings when assessing the lung parenchyma by TU are due to processes occurring at the level of the pleural line or peripheral lung involvement, caused by underlying disease.
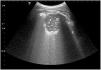
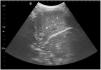
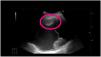
In the diagnosis of pneumonia, different ultrasound signs must be considered, which may point to the existence of a consolidated infection or other non-infectious diseases. These signs are found using air bronchograms, with hyperechogenic lines within the consolidation due to the posterior reinforcement produced by the air bronchogram in pneumonia, static and dynamic air bronchogram, and irregular marginal contour. The vascular pattern can also be assessed, by noting the existence of colour within the consolidation when using colour Doppler. Another finding may be the simple existence of an increase of B-lines in an area of the scanned chest, as a peripheral manifestation of underlying pneumonia (Fig. 3). Also, the presence of a consolidation with the presence of the liquid bronchogram sign should suggest obstructive atelectasis30–32 (Fig. 4). Currently, because TU requires trained personnel and has not been standardized, it is not included in clinical practice guidelines as a standard technique and is not used as an alternative to chest X-ray or chest CT.33
Infectious consolidation. Chest ultrasound performed with a convex probe and in longitudinal projection showing infectious consolidation in contact with the chest wall and the presence of air bronchogram inside (A), pleural line (B) which is abruptly interrupted by the presence of consolidation and an increase in B-lines (C).
Atelectasis of obstructive origin. Thoracic ultrasound performed with a convex probe and in transverse projection showing homogeneous and hypoechogenic consolidation (A) with the presence of anechogenic lines in its interior, branched (B) and without colour Doppler signal called fluid bronchogram sign.
The presence of B-lines represents one of the most frequent findings and can be related to many pulmonary diseases including heart failure, interstitial lung disease, pneumonia, pulmonary contusions, lymphangitis and COVID19. For this reason, we must always consider the clinical context of each patient we treat.34
In interstitial lung diseases, there is a pathological increase in B-lines, pleural thickening, irregularity and destructuring of the pleural line, with bilateral and predominant involvement of the lower lobes. However, these findings do not provide information about the degree of disease involvement; an attempt to fill this gap is made by the Shear Wave Elastography (SWE) method, which relates ultrasound wave conduction velocity and the degree of elasticity to the degree of pulmonary fibrosis.35–37
With the advent of COVID19, the use of TU has increased for the study of lung involvement. A recently published systematic review of this disease shows a predominance of confluent B-lines, pleural thickening, and irregularity, subpleural consolidations and bilateral distribution with predominance in posterior and lower fields. Pleural effusion is present in severe COVID19 infections.38
A recent meta-analysis found that the COVID19 ultrasound score was associated with mortality and disease severity, being higher in the lowest survival group. However, there is no standardized type of ultrasound probe, number of lung fields to be analyzed or reference severity index, although it has been observed that it can be used as a tool in monitoring disease severity at the bedside.39
Chest Wall MediastinumThe chest wall is the first structure encountered when performing a lung ultrasound and it is important to understand its properties because of the impact they can have on the image obtained. To optimize the visibility of the B-lines, a higher gain and focus on the pleural line is required.40 However, there is interpersonal variation, due to the different thickness of panniculus adiposus and musculature, which does not allow the results to be generalized. For all these reasons, in order to standardize criteria for ultrasound image improvement, it would be necessary to study the tissue behaviour of ultrasound in the different structures.
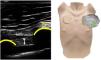
The intercostal muscles play an important role in the movement of the rib cage, especially in cases where the function of the diaphragm is impaired or the rate of breathing increases. Their thickening fraction can be measured at the parasternal level, with a linear probe placed in the sagittal plane, 3cm from the sternum between the 2nd and 3rd ribs,41 and this has proven to be a useful tool for predicting weaning in mechanically ventilated patients42 (Fig. 5). The intercostal thickening fraction (TFic) in healthy patients is <5% basally and increases with increasing respiratory demand.42,43 Increases in TFic >30% have been associated with diaphragmatic dysfunction and weaning failure,42,43 whereas TFic <11.5% is associated with successful weaning with an S: 86.9% and E: 85%.44 In COPD patients, diaphragmatic thickness and echogenicity have been related to FEV1%45 and the risk of exacerbations.46 Measurement of these parameters in the intercostal musculature is more accessible than assessment of the diaphragmatic musculature and is particularly useful in case of dysfunction of the latter.47
The figure shows a B-mode ultrasound of the parasternal intercostal muscle. The position of the probe is in the parasternal space between the 2nd and 3rd ribs. The intercostal muscle is located between two ribs, cranial to the pleural line and behind the pectoralis muscle.
However, neither thickness nor TFic assesses longitudinal muscle fibre shortening. Speckle tracking permits effective measurement of tissue deformation, and its use at the level of the intercostal muscles has shown greater predictive value for successful weaning compared to conventional parameters.48
Wall lesions account for 5% of thoracic tumours, and to improve their characterization and optimize sampling, new scanning techniques such as contrast-enhanced ultrasound (CEUS) are being developed.49,50 Chest wall lesions present a systemic perfusion pattern, so the main utility of CEUS in these lesions is to optimize sampling by improving the visualization of vessels and lesion borders, and avoiding necrotic areas.51,52
Mediastinal ultrasound has mainly focused on the evaluation of masses and visualization of the heart and pericardium, as well as lymph node staging using echo bronchoscopy. However, this tool has been used little for assessing other mediastinal structures, such as the upper airway. Ultrasound can be useful for detecting vocal cord paralysis, identifying the crico-thyroid membrane, and verifying that orotracheal intubation has been performed correctly53 (Fig. 6).
(A) Longitudinal assessment, superior with linear probe. Cranially we find the thyroid cartilage (red), posteriorly the cricoid cartilage (blue), between the two the crico-thyroid membrane. Caudally we find the tracheal rings (yellow) and just above them the thyroid isthmus (shaded orange). (B) Transversal assessment, upper with linear probe. The thyroid cartilage (red) is found in the most superficial area, followed by the vocal cords (over-surrounded) and in depth by the arytenoid cartilage (pink). (C) Transverse assessment, lower with linear probe. The double yellow line represents the trachea. The oesophagus is shaded in the posterior area, but does not differentiate the double hyperechogenic line. If a double line is visible at oesophageal level, we must consider that the orotracheal intubation has not been performed correctly. Image created by the author.
Echobronchoscopy permits identification of underlying structures on the other side of the tracheal/bronchial wall.
Radial echo bronchoscopy (radial EBUS) uses very fine mini-ultrasound probes that are introduced through the wide channel bronchoscope. These probes are transducers that are rotated by an external motor with a frequency of 20MHz (between 12 and 30MHz). They can be central, to assess neoplastic or other central airway involvement, or peripheral (1.4–1.7mm diameter), for more distal lesions. The latter is the main indication. The probe is introduced distally and provides a 360° image of the adjacent structures, which is usually described as a snowstorm, however, once positioned in the solid lesion, the image shows a hypoechogenic area with enhancement, bordering the normal lung. This technique shows superior cost-effectiveness in sampling compared to fluoroscopically directed transbronchial biopsy. Modifications of the technique to perform it without fluoroscopy or with the use of a cryotherapy probe are currently under study.54,55
Linear bronchoscopy is performed using an ultrasound transducer attached to the end of a bronchoscope. It allows us to study the mediastinum and pulmonary hilaris. EBUS-guided transtracheal or transbronchial puncture (EBUS-TBNA) permits minimally invasive staging56 and restaging of patients with non-small cell lung cancer (NSCLC),57 as well as the diagnosis of adenopathies and hiliomediastinal masses related to metastases of extrathoracic neoplasms,58 as well as processes such as lymphomas,59 sarcoidosis60 and treatment-induced sarcoid-like reaction.61,62 This technique is a clear alternative to mediastinoscopy in the staging of NSCLC, and given its high sensitivity, low risk profile and cost-effectiveness,63 it is the first technique indicated in all clinical reference guidelines for the study of both neoplastic and non-neoplastic hiliomediastinal pathology.64
When performing the technique, a systematic assessment of the different stations should be made, and the description should refer to the number of nodes per station, size and ultrasonographic characteristics (grey scale combined with vascular distribution pattern), as accepted in multiple studies (Fig. 7) although their diagnostic utility for predicting metastatic infiltration still needs validation and assessment.65–69
Ultrasonographic features during EBUS used to differentiate metastatic adenopathies from those of reactive origin: size, axis (long axis/short axis), shape (oval/round), margin (clear/not clear), central hilar structure (present/absent), echogenicity (homogeneous/heterogeneous), necrosis (present/absent), conglomeration (present/absent), calcification (present or absent), vascular pattern (avascular/hilar/non-hilar/capsular/centrally, mixed). Rounded shape, absence of central hilar structure, presence of conglomerate and non-hilar vascular perfusion pattern may predict lymph node metastasis.
Several technique-related factors can influence the diagnostic yield,70 and sampling should be as systematic as possible. There is a general consensus that the minimum number of entries per nodal station should be three, although the influence of other factors such as type of needle, mode of aspiration and means of fixation is still under study. Supplementation with EUS increases sensitivity by 10%, as has been shown in several studies.71
Elastography-based imaging techniques have received substantial attention in recent years for non-invasive assessment of tissue mechanical properties. These techniques take advantage of changed soft tissue elasticity in various pathologies to yield qualitative and quantitative information that can be used for diagnostic purposes. Measurements are acquired in specialized imaging modes that can detect tissue stiffness in response to an applied mechanical force (compression or shear wave). Ultrasound-based methods are of particular interest due to its many inherent advantages, such as wide availability including at the bedside and relatively low cost. The EBUS elastography has started to be used recently. There is not yet a specific protocol for image acquisition for EBUS elastography measurements, it has been carried over from other protocols (e.g. liver).72
A recent meta-analysis73 analyzed seventeen studies with 2307 lymph nodes analyzed. There was significant heterogeneity across the included studies. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and diagnostic odds ratio for the diagnosis of hilar and mediastinal LNs by EBUS elastography were 0.90 (95% confidence interval [CI], 0.84–0.94), 0.78 (95% CI, 0.74–0.81), 4.1 (95% CI, 3.4–4.9), 0.12 (95% CI, 0.07–0.21) and 33 (95% CI, 17–64), respectively. Furthermore, area under the curve was calculated to be 0.86 (95% CI, 0.82–0.88). This study concludes that EBUS elastography is a valuable technology in the differentiation of benign and malignant hilar and mediastinal nodes and could provide supplementary diagnostic information during endobronchial ultrasound-guided transbronchial needle aspiration. The combination of EBUS elastography and B-mode EBUS could improve the diagnostic accuracy for hilar and mediastinal nodes. At present, this technique cannot replace biopsy, but it provides information on suspicious areas that can be biopsied, thus increasing the diagnostic yield of biopsy. More studies are needed to evaluate the usefulness of this technique, with larger series of patients.
Ultrasound-guided InterventionsTU has proven to be highly useful for performing procedures, both in the pleural space and in the lung parenchyma, whenever lesions are in contact with parietal pleura. In these lesions, TU is just as effective as computed tomography as a guide for performing procedures but has a shorter execution time and lower cost and produces less radiation.
TU is used prior to thoracentesis to identify the best site for the procedure and to measure the depth of adjacent organs to avoid puncturing them. This reduces the number of pneumothoraces when compared to thoracic percussion. It is also used after thoracentesis to assess normal lung sliding and to rule out iatrogenic pneumothorax.74,75 The ultrasound appearance of the pleural fluid will guide the decision to initiate intrapleural fibrinolytic therapy.76–78
Ultrasound-guided pleural biopsy should be considered the first-line approach when diagnosing pleural effusion with suspected malignancy or tuberculosis.79 Pleural thickness above 20mm increases the diagnostic yield to almost 100% (Fig. 8). In comparison with CT-guided biopsy, in addition to the well-known advantages of speed and absence of radiation, the operator can compensate for the patient's respiratory movements in real time without the need for apnea manoeuvres.80
Only lung lesions in the lung periphery which are in contact with the parietal pleura will be accessible by TU. Compared to CT, some studies recommend the use of ultrasound in lesions larger than 10mm, with an overall yield of more than 90%, with the surface area in contact with the pleura having more influence than the diameter of the lesion.81 The best results are obtained when puncturing lesions with over 30mm of pleural contact, and puncturing nodules smaller than 10mm should be avoided.82 In terms of size, yield decreases below 20mm, due to technical difficulties, and above 50mm, in the latter case due to the progressive increase in the percentage of necrosis with increasing lesion size.83 This problem of reduced yield due to necrosis can be solved using elastography, which enables us to differentiate the areas of necrosis, allowing us to direct the puncture or biopsy towards the areas of greatest diagnostic yield (Fig. 9). In these cases, the application of ultrasound contrast could also be useful as a puncture guide, since its application allows us to detect the presence of necrosis within the lung lesions with greater precision, thus increasing the yield.84
Subpleural lung neoplasia. The image on the left (A) corresponds to the application of elastography on the pathological image. The “blue” part indicates the non-necrotic tumour area. In the image on the right (B), the ultrasound guide is directed to the tumour area corresponding to the “blue” colour of the elastography image.
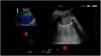
TU is a basic application of critical ultrasound that associates urgent diagnoses with immediate therapeutic decisions. The IRCU uses the BLUE protocol, a rapid protocol (<3min) that allows the diagnosis of acute respiratory failure85 (Fig. 1).
In the ventilated patient, the ultrasound is a tool to guide and monitor mechanical ventilation.86,87 It allows visualization of the orotracheal tube and to rule out selective bronchial placement.88 It allows guiding the adjustment of mechanical ventilation.89 Aeration during a recruitment manoeuvre can be visualized directly and in real time.90 If there is anteroposterior collapse, positive end-expiratory pression (PEEP) is often useful, but when there is no anterior collapse there is a risk of overdistension.91 PEEP-induced end-expiratory lung volume can be estimated in real time.92 If using PEEP, there is a mobile air bronchogram, it is better and less risky to use ventilation than bronchoscopy to recruit atelectasis tissue.93
Patients classified as non-responders to PEEP may successfully respond to prone. In addition, the amount of dorsal aeration on prone is associated with a positive clinical response, defined as a PaO2/FiO2 (PaFi) greater than 300mmHg after 7 days of treatment.94 Ultrasound can also be used to predict MV weaning failure and its cause (unresolved lung disease, diaphragm dysfunction, heart failure, …).95
Training in FAST (Focused Assessment with Sonography in Trauma) and E-FAST (Extended Focused Assessment with Sonography in Trauma) lung ultrasound is required in the IRCU,96 as well as in echocardiography.
The primary windows used (Fig. 10) include the following:
- 1.
The anterior thoracic view. As the pleura normally slides easily, the absence of this sliding and possible separation of the pleura by a pneumothorax can typically be visualized in the second or third intercostal space with a high frequency transducer. The identification of a pulmonary point is a highly specific way to diagnose pneumothorax and represents the site where the lung is attached to the parietal pleura immediately adjacent to the pneumothorax. The full chest scan97 enables us to diagnose PD, pachypleuritis, alveolar syndrome, interstitial syndrome, lung masses, extrathoracic lesions, and a ruled echocardiography. There are several, varying, scores of usefulness. For weaning, knowledge of diaphragmatic ultrasonography is essential.98,99
- 2.
The right upper quadrant view (also known as the perihepatic, Morrison's pouch or right flank view) uses the liver as an ultrasound window to assess the liver and the hepatorenal space (Morrison's pouch) for free fluid. A slight cephalic movement of the transducer permits imaging of the right pleural space for free fluid. Movement of the caudal probe allows us to visualize the lower pole of the right kidney, as well as the right paracolic space for free fluid assessment.
- 3.
The left upper quadrant view (also known as perisplenic or left flank view) uses the spleen as a window to examine the spleen and the perisplenic space above the spleen, below the diaphragm and the splenorenal recess. The cephalic scan permits visualization of the left pleural space. The caudal scan permits visualization of the lower pole of the left kidney and the left paracolic space.
- 4.
Pelvic view (also known as retrovesical, retrouterine or Douglas pouch view) enables us to assess the space which is most dependent on the peritoneum for free fluid. When free fluid is present, it is most often seen posterior or superior to the bladder and uterus.
- 5.
The pericardial view (also known as the subcostal or subxiphoid view) uses the left lobe of the liver as an acoustic window to allow us to analyze the heart, particularly its right side. Both sagittal and transverse 4-chamber planes can be used. The space is scanned for free pericardial fluid at anterior or posterior locations. A slight posterior or inferior angulation in this view allows us to visualize the IVC (inferior vena cava) and hepatic veins, including their normal respiratory variability. Assessment of the IVC is particularly useful in hypovolaemic patients (e.g., secondary to massive haemorrhage) or those with severe fluid overload.
BLUE protocol. This algorithm (Chest 2008;134:117–125), is a proposal for the immediate diagnosis of the main causes of acute respiratory failure, using a pulmonary and venous ultrasound approach. Profile A associates anterior lung slippage with A lines. Profile A′ is an A-profile with suppressed pulmonary sliding. Profile B associates the anterior lung sliding with B-lines. Profile B′ is a B profile with suppressed lung sliding. The C-profile indicates anterior lung consolidation, regardless of size and number. An irregular and thickened pleural line is its equivalent. A/B profile is half an A profile in one lung and half a B profile in the other.
The development of new techniques, such as contrast, has shown that they have a key present and future role to play in the field of chest pathologies.
The presence of a double arterial supply in the lung from the bronchial and pulmonary arteries makes it an ideal organ for study by contrast-enhanced ultrasound. The work of Sartori et al. showed that late uptake of peripheral pulmonary consolidation was the criterion with the highest sensitivity, specificity, and positive predictive value for the diagnosis of malignancy.100 The evaluation of the different uptake parameters (time to uptake, contrast washout, type of uptake, extent of uptake, homogeneity, …) permits a precise differential diagnosis of peripheral lung consolidations to be established101 (Fig. 11). Currently, certain characteristics of contrast uptake are considered extremely useful in determining the benign or malignant nature of a peripheral lung mass, or its consolidation (Table 1).102
Typical Findings on Contrast-enhanced Lung Ultrasound that Allow Differentiation Between Benignity and Malignancy.
| Typical Findings | Benignity | Malignancy |
|---|---|---|
| Capture mode | Dendritic | Centripetal |
| Type of internal recruitment | Uniform | Non-uniform |
| Time to recruitment | Precoz | Late |
| Time difference between the arrival of contrast to the healthy lung and to the lesion | <2.5s | ≥2.5s |
The application of ultrasound contrast to guide lung biopsies has been shown to substantially increase the success rate of the test due to an increased ability to detect necrosis within the lung.103–106 The detection of necrosis inside suspicious lung lesions is of particular importance in those which are larger than 5 centimetres.51 In addition, the incorporation of contrast prior to biopsy increases the yield of the test by providing a higher number and proportion of tumour cells, and higher quality cells.107
Despite the great diagnostic utility of using contrast to study pulmonary consolidation, the presence of a single vascularization does not allow us to make a narrow differential diagnosis in the study of mediastinal pathologies. However, the high capacity for detecting necrosis makes contrast-enhanced ultrasound an ideal technique for ultrasound-guided biopsies.108–110 Regarding pleural pathology, the first published articles indicate that there appear to be some parameters that can discriminate between malignancy and benignity.111 However, prospective studies are needed to determine the real usefulness of contrast-enhanced ultrasound in this field. Pleural biopsy also benefits from the application of ultrasound contrast because it allows us to detect necrosis more reliably and correctly visualizes the thoracic vessels, thus avoiding their injury during the procedure (Figs. 12 and 13).112,113
Actinomycosis of the lung with involvement of the left chest wall. (A) T1-weighted chest MRI after intravenous gadolinium administration. (B) T2-weighted thoracic MRI with fat suppression. (C) Contrast-enhanced ultrasound images after 4s after injection (C1), 6s (C2), 10s (C3), 14s (C4), 20s (C5), 36s (C6), 45s (C7) and 60s (C8). The images show a voluminous lung lesion with extensive involvement of the chest wall (white star) showing absence of early enhancement after administration of ultrasound contrast (C1 and C2), despite evidence of uptake in the adjacent parenchyma (red arrows) and within the right (white arrows) and left ventricles (yellow arrow). The lesion shows arterial supply from chest wall arteries (green arrows) and progressive homogeneous uptake in the systemic arterial phase together with slow washout. A small area of necrosis can be seen in the pulmonary part of the infection (blue arrows).
Pleural tuberculosis. (A) Conventional chest ultrasound showing left pleural effusion (white star) and irregular thickening of the diaphragmatic pleura (white arrow). (B) Contrast-enhanced ultrasound 20s after injection showing uptake of the irregular thickening of the diaphragmatic pleura and lateral costophrenic sinus (red arrows) which is more evident than in the conventional scan. (C) Contrast-enhanced ultrasound 45s after injection showing some washout of contrast (red arrows) and some focus of necrosis within the pleural thickening (green arrow). (D) Ultrasound-guided pleural biopsy showing the needle (blue arrows) pointing towards the diaphragmatic pleural thickening.
TU is a highly useful technique in the diagnosis and management of different thoracic diseases. It is superior to other radiological techniques (CT, simple radiology) in the assessment of some entities, and the information provided by the different techniques is complementary in most cases.
Its use as a guide in interventional procedures increases safety of the different examinations.
The introduction of new techniques, such as contrast and elastography, increases the effectiveness of scanning in most diseases, but prospective studies are needed to assess its actual usefulness in some entities.
The use of TE is now becoming much more widespread among pneumologists. The specialized training received, together with the different ultrasound patterns, allow the technique to be performed safely.
Conflict of InterestsThe authors state that they have no conflict of interests.