Despite wide antiretroviral scale-up during the past two decades resulting in declining new infections and mortality globally, HIV-associated tuberculosis remains as a major public health concern. Tuberculosis is the leading HIV-associated opportunistic infection and the main cause of death globally and, particularly, in resource-limited settings. Several challenges exist regarding diagnosis, global implementation of latent tuberculosis treatment, management of active tuberculosis, delivery of optimal patient-centered TB and HIV prevention and care in high burden countries. In this article we review the advances on pathogenesis, diagnosis, and treatment after nearly two decades of global roll-out of antiretroviral therapy and discuss the current challenges for the global control of tuberculosis-HIV co-infection.
A pesar de que el uso de antirretrovirales ha aumentado en uso y difusión durante las 2 últimas décadas, lo que ha resultado en la disminución de nuevas infecciones y de la mortalidad global, la tuberculosis asociada al VIH sigue siendo un importante problema de salud pública. La tuberculosis es la principal infección oportunista asociada al VIH y la principal causa de muerte a nivel mundial, particularmente en el marco de situaciones de recursos limitados. Existen varios retos con respecto al diagnóstico, la implementación global del tratamiento de la tuberculosis latente, el manejo de la tuberculosis activa, el proporcionar una prevención óptima de la tuberculosis y el VIH centrada en el paciente y la atención en países de alta carga. En este artículo revisamos los avances en la patogénesis, el diagnóstico y el tratamiento después de casi 2 décadas de implementación global de la terapia antirretroviral y comentamos los retos actuales para el control global de la coinfección tuberculosis-VIH.
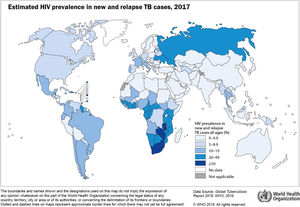
Worldwide, tuberculosis (TB) is the leading cause of death from a single pathogen. According to the World Health Organization (WHO), about 10 million developed active tuberculosis disease and 1.6 million people died in 2017.1 Globally, HIV-associated TB was estimated to be responsible for about 9% of all new cases and 20% of all TB-deaths.1 Encouragingly, the prevalence of HIV among new TB cases is slowly decreasing in recent years. Nevertheless, it is still unacceptably high in sub-Saharan Africa due to several challenges.1 Seventy-two per cent of all TB cases among people living with HIV (PLHIV) occurred in Africa (Fig. 1). The global burden of disease study estimates a slightly lower number of TB cases and less TB-deaths, especially among PLHIV,2 reflecting under-diagnosis and under-reporting of TB, a major obstacle to estimate the true number of new cases. Indeed, of the 920,000 HIV-associated TB cases estimated last year by the WHO, only 51% had been diagnosed and/or reported. Reducing this diagnostic gap is a priority of the global End TB strategy.1 PLHIV, including those receiving antiretroviral treatment (ART), have a 30–35 higher risk of developing3 and dying of TB4 than HIV-negative individuals. It is therefore paramount that TB patients starting ART are routinely tested for HIV. Unfortunately, despite increasing yearly, the proportion of notified TB cases with a documented HIV result in 2017 was only of 60%.1
In this article we review the advances on pathogenesis, diagnosis, treatment, and current challenges for the global control of TB-HIV co-infection.
Pathogenesis of tuberculosisMycobacterium Tuberculosis (MTB) is transmitted by droplet nuclei expelled from the respiratory tract of infected individuals through coughing and, less frequently, through sneezing, laughing and singing.5–7 Inhalation and deposition of infected droplet nuclei in the host lung triggers infection,5,8 where it is recognized and phagocytosed by alveolar macrophages.6,8,9 Subsequent course of infection, determined by MTB virulence and the host immune response, is mediated by recruitment and activation of alveolar macrophages. Inability of alveolar macrophages to destroy or inhibit MTB results in intracellular proliferation of the pathogen, macrophage cell death and pathogen release, which induces a repeat cycle aimed at limiting further bacterial growth.9,10 MTB infected alveolar macrophages secrete cytokines and chemokines that initiate an inflammatory cascade, attracting other phagocytic cells to form a granuloma, the hallmark of TB disease,5,8,10 intended to wall-off the pathogen and limit its spread to other organs. Inability of the host immune response to adequately control bacterial replication following first infection with MTB results in primary TB disease. This either heals with persistence of viable organisms or progresses to active TB disease. Erosion of caseous necrotic lesions into airways causing mechanical spread of bacilli results in infectiousness,5 whereas systemic infection results from migration of TB-infected cells into the lymphatic and circulatory systems.5,6,10 While focal lesions are typical of TB, lung biopsy studies demonstrate parenchymal pathology ranging from pulmonary infiltration with an exudative reaction, granulomatous inflammation, fibro-caseous disease,5,8 to cavities resulting from necrosis of tuberculous pneumonia.
Post-primary TB disease occurs by reactivation of dormant organisms from previous primary TB infection, or from exogenous re-infection. While a granulomatous process is seen in primary tuberculosis, pathology in post-primary TB follows a pneumonic process characterized by accumulation of foamy macrophages in alveoli of lung apices with acid fast bacilli organisms seen almost exclusively in alveolar macrophages. In high bacillary burden disease, caseous necrosis may evolve into cavities, which may progress slowly for up to a year producing little or no clinical illness.
Active TB disease develops in approximately 5% of MTB-infected individuals within two years. In 90–95%, dormant viable bacteria survive for years, progressing to active disease at a rate of 10% per lifetime and 10% per annum in HIV-uninfected and PLHIV respectively.11,12 The innate immune response through B cells and antibodies mediate MTB killing directly through stimulation of antigen presentation, and cytokine production aimed at enhanced killing of MTB infected macrophages. Quantitative and qualitative depletion of CD4 T-cells during HIV infection increases the risk of MTB disease. Characteristically, this immunologic impairment is associated with impaired granuloma formation, compromised MTB containment and increasing bacillary burden.11,13,14 Indeed, the HIV epidemic has played an important role in increasing the prevalence of miliary and extrapulmonary TB globally. In turn, TB enhances HIV viral replication by increasing expression of viral growth receptors such as CXCR4.15 Reactivation of latent TB infection is likely precipitated by disruption of the stable host-microbe interaction.15 In PLHIV, multiple factors including diversity in lung microenvironments, levels of immune activation and cytokine secretion, and variation in granuloma genesis, cumulatively expose MTB to varying levels of nutrients and oxygen that promote or prohibit replication, influence TB pathogenesis and disease severity.16
Diagnosis of tuberculosis among people living with HIVDiagnosis of latent tuberculosisTwo major tests exist for the diagnosis of latent tuberculosis infection: the tuberculin skin test (TST) and the interferon-gamma release assays (IGRA).17 A thorough review of these two types of tests is outside the scope of this article. All newly diagnosed PLHIV should be screened for latent tuberculosis with TST or IGRA.18,19 Also, those patients tested negative while having low CD4 counts (<200cells/μL), should be retested after immune reconstitution above this threshold.18,20 IGRA is generally preferable, if available, particularly for patients with prior vaccination with Bacille Calmette-Guérin (BCG) and for patients who are unlikely to return to have the TST read. It is reasonable to confirm a positive TST result with an IGRA in low TB incidence settings, to rule out nontuberculous mycobacterial infection.21 For those who test negative with ongoing risk factors for active TB, annual screening is warranted. Testing should not be repeated among those with a previously positive test. Treatment should be administered to those with a positive TST or IGRA results after having excluded active TB. In high prevalence settings where TST or IGRA are often not available, treatment of latent tuberculosis infection is recommended to all PLHIV regardless of testing results, after having excluded active TB.22
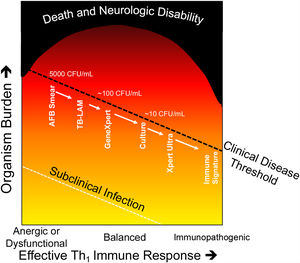
Diagnosis of active tuberculosis diseaseDiagnosis of active TB disease in PLHIV is challenging due to the differing and non-specific clinical symptoms, and often healthcare workers rely on suggestive radiological changes or clinical suspicion.23,24 The level of HIV-associated immunosuppression and the resulting TB burden influences the yield of the different available diagnostic methods, including smear microscopy, culture, PCR, urine lipoarabinomannan, and whole-genome sequencing (Fig. 2).
Damage response framework in relation to Tuberculosis.
Modified from the damage–response framework of microbial pathogenesis. (24). As the immune response is less effective due to HIV-infection, energy or dysfunctional responses (e.g. Th2 or neutrophil response) immune control of the pathogen is less effective, and TB bacilli are more easily detected by microbiologic diagnostics. As a more effective Th1immune response occurs, less viable TB bacilli exist and diagnosis by traditional techniques becomes more challenging. In the extreme, such as in paradoxical immune reconstitution inflammatory syndrome, immunopathology can occur in the absence of viable or detectable organisms. Mortality is highest in those with the most attenuated or exuberant inflammatory responses.
Historically, diagnosis of TB disease has relied on microscopy (Ziehl-Neelsen staining of Acid-Fast Bacilli) of fluid or tissue from TB-affected organs. Although widely available in laboratories globally, the limited sensitivity of traditional microscopy: ∼50% sensitive for high bacterial burden TB disease (such as cavitary PTB in adults) and 10–20% sensitive in paucibacillary disease (such as TB-meningitis), has limited its clinical utility.25
CultureCulture of clinical specimens for MTB has a 50–100% sensitivity depending on bacillary load and the clinical specimen tested. MTB culture techniques however: are expensive ($13–$50/unit); are not universally available; require a biosafety level-3 laboratory; and results takes at least 10 days in liquid media (Mycobacteria Growth Inhibitor Tube culture) and up to 8 weeks on solid media (Löwenstein-Jensen), too slow to aid clinical decision making.
Commercially available PCR platformsThe introduction of the GeneXpert® MTB/Rif (Xpert) assay (Cepheid, Sunnyvale, CA, USA) a cartridge-based, real-time PCR molecular assay in 2010 marked a new era in TB diagnostics. Xpert MTB/RIF has notable benefits over traditional microbiology techniques: Xpert MTB/RIF is fully automated and requires minimal operator training; a biosafety level-3 laboratory is not required; and the run-time of ∼2h enables rapid results turnaround of ∼48h. In addition, Xpert MTB/RIF provides preliminary drug sensitivity testing (DST) through detection of rpoB gene mutations conferring rifampicin resistance. In a 2014 Cochrane review evaluating the role of Xpert MTB/RIF in diagnosing PTB, pooled sensitivity and specificity was 89% (95% CI 85–92%) and 99% (95% CI 98–99%), respectively.26 In 2011, the WHO issued a strong recommendation for Xpert MTB/RIF to be used as the preferred initial diagnostic test for sputum samples in diagnosing PTB27; followed in 2013, by a recommendation for use with CSF specimens, lymph nodes and other tissues in diagnosing EPTB.26 However, the suboptimal sensitivity of Xpert MTB/RIF in paucibacillary disease and among PLHIV is a significant limitation. Pooled sensitivity from seven studies in PLHIV was 79% (95% CI 70–86%).26 In 2017, Cepheid therefore introduced GeneXpert® MTB/Rif Ultra (Ultra) a re-engineered version of the Xpert cartridge MTB/RIF platform with technical enhancements, including larger specimen volume, additional probes for two other DNA targets in the mycobacterial genome, optimized fluidics and PCR cycling and addition of a’trace’ category for the lowest bacillary load. The improved sensitivity of Ultra is purported to have greatest clinical utility in cohorts of PLHIV. In a multi-center, diagnostic accuracy study, comparing Xpert Ultra and Xpert MTB/RIF in PTB diagnosis, Ultra sensitivity demonstrated a greater additional diagnostic benefit in PLHIV: 13% difference (95% CI 6.4–21%) compared to 1.3% (95% CI 1.8–4.9%) improvement in HIV-uninfected patients.28 A recent study conducted in Uganda showed a sensitivity of Xpert Ultra of 95% for diagnosing tuberculous meningitis (TBM), which was higher than Xpert MTB/RIF (45%) and culture (45%).29 Based on this highly improved sensitivity, the WHO has endorsed Ultra,30 including for TBM, and it has been made available to eligible countries at the same concessional pricing as the standard Xpert MTB/RIF cartridge.
Urine lipoarabinomannan (LAM) antigen detectionThe increasing recognition and availability of urine lipoarabinomannan (LAM) antigen detection lateral flow assays marked a significant step forward in the diagnosis of disseminated HIV-associated TB. Urine is easily obtained without risk of aerosolization of MTB and results are available in 20min, facilitating timely clinical decision making. The sensitivity of LAM is only moderate (pooled sensitivity and specificity across six studies was 44% (95% 31–60%) and 92% (95% 83–96%), respectively31 but its sensitivity is greatest in those with a CD4 count <100cells/μl. Moreover, TB-LAM has recently been shown to have demonstrable clinical benefit including increased overall TB diagnoses and significant reduction in mortality in high risk subgroups: CD4 <100cells/μL; and hemoglobin <8g/dl when hospitalized PLHIV underwent systematic screening.32 TB-LAM LFAs are complementary to other diagnostics and at ∼$4 per test are potentially a cost-effective intervention when used in the appropriate population.
Whole-genome sequencingWhole genome sequencing of MTB for detection of drug resistance and typing is increasingly being used in a range of clinical and research settings. The recently demonstrated capacity to sequence MTB directly from clinical specimens33 has the potential to revolutionize the TB diagnosis and DST in both high- and low-income countries.34
Treatment of tuberculosis-HIV co-infectionTreatment of latent tuberculosisIsoniazid preventive therapy (IPT) has the potential to deliver greater health benefits to PLHIV in high-incidence countries.35 In the TEMPRANO study, a trial designed to assess the benefits of early antiretroviral therapy (ART), 6-month IPT, or both among adult PLHIV with high CD4+cell counts, immediate ART and 6-month IPT independently decreased the risk of death or HIV-related severe illness compared to deferred ART and no IPT.36 Despite its efficacy, several challenges exist hampering the wide roll-out of IPT. Costs, length of treatment and pill burden difficult its implementation and hamper adherence. Moreover, the incidence of TB in sub-Saharan Africa may increase soon after IPT discontinuation.37 As a result of these challenges, treatment of latent TB is seldomly available in resource-limited settings (RLS), with only one third of countries currently implementing it among PLHIV.1
Shorter treatments, such as the newly recommended weekly high dose isoniazid and rifapentine regimen for12 weeks (3HP),38 which have shown to increase adherence to TB preventive treatment39 may contribute to a higher implementation in RLS. However, the duration of protection of 3HP is still being evaluated in the WHIP3TB trial (https://clinicaltrials.gov/ct2/show/NCT02980016). Also, availability and authorization of rifapentine is not equally distributed throughout the world. Recently, a combination of daily rifapentine and isoniazid (1HP) for only one month, has been evaluated in the BRIEF trial, showing fewer adverse events and higher adherence compared to 9-months of isoniazid.40 The implementation of these regimens in low endemic areas is hampered by the low availability of rifapentine. However, there is already some evidence of a better completion rates and fewer adverse events when using 3HP.41
Treatment of active TB-HIV co-infectionEarly initiation of anti-TB therapy and ART for patients with HIV-associated TB is critical in reducing morbidity and mortality as well as reducing onwards transmission of both infections; several important management challenges however exist: (i) when to start ART; (ii) significant PK drug-drug interactions (DDI); (iii) additive toxicities of concomitant ART and anti-TB drugs; and (iv) TB-associated immune reconstitution inflammatory syndrome (TB-IRIS). The global HIV response serves as one of the most inspiring undertakings in the history of global health. ART roll-out has been impressive, with around 24 million people now on ART globally. Several countries worldwide, including Botswana, have met or are on track to meet the UNAIDS 90:90:90 targets (90% of people living with HIV knowing their status; 90% of those on ART; and 90% of people receiving ART virologically suppressed by 2020).42 Conversely, TB drug development and roll out has moved more slowly, with no new first-line agents since the 1970s and pill burden and side-effects remaining a huge challenge. It is therefore perhaps not surprising that TB mortality, particularly in HIV co-infection, has failed to decline in line with End-TB targets. In addition, multi-drug resistant TB (MDR-TB) poses a growing challenge in many regions.1,43 Bedaquiline has been WHO-approved for the treatment of drug-resistant TB for 5 years and will hopefully replace injectable agents (which can cause deafness and kidney injury) but uptake has been slower than anticipated.44 Clinical trials are currently underway investigating an all oral short course (6–9-month) regimen for multi-drug (MDR) and extensively-drug resistant (XDR) TB, a welcome alternative to the existing protracted regimen including injectable agents. The nitroimidazoles, delamanid and pretomanid, are less toxic and a useful addition to the armamentarium for the treatment of drug-resistant TB. Linezolid, combined with bedaquiline and pretomanid during six months have demonstrated a high success rate, it is safe and can change the paradigm of the therapy of XDR TB.45 A number of other novel agents are in the development pipeline (https://www.newtbdrugs.org) and higher dose of rifampicin and isoniazid are also being explored as a way of shortening the treatment duration (https://clinicaltrials.gov/ct2/show/NCT02581527).
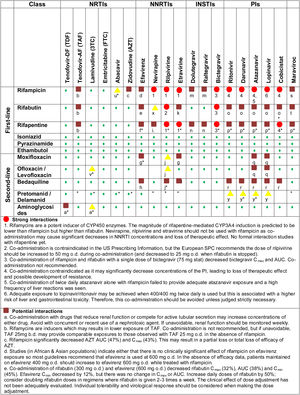
TB-HIV drug-drug interactionsRifamycins, the cornerstone of treatment for drug sensitive TB, are associated with a considerable potential for PK DDI. Rifampicin is a potent inducer of the hepatic CYP450 enzymes (including CYP2A6, CYP2B6, CYP2C, and CYP3A isoenzymes), P-glycoprotein (P-gp), and uridine diphosphate glucuronosyltransferase (UGT) 1A1 enzymes.46 The magnitude of rifapentine-mediated CYP3A4 induction is predicted to be lower than rifampicin but higher than rifabutin. This induction alters pharmacokinetics of drugs metabolized by these pathways, reducing plasma concentrations of several antiretroviral drugs, which risks loss of efficacy and sequential development of resistance mutations. As a potent enzyme inducer, rifampicin is not recommended for use in patients receiving protease inhibitors (PI), certain non-nucleoside reverse transcriptase inhibitors (NNRTI) such as rilpivirine, nevirapine, and etravirine; some NRTI such as tenofovir-alafenamide; and some integrase strand transfer inhibitors (INSTI), such as elvitegravir and bictegravir. Rifabutin, a weaker CYP3A4 enzyme inducer is recommended as an alternative to rifampicin in patients receiving PI-based ART regimens. Because rifabutin is a substrate of the CYP450 enzyme system however, its metabolism may be affected by NNRTIs or PIs and rifabutin dosage adjustment is generally recommended (Fig. 3). Efavirenz-based ART regimen is recommended as the preferred first-line regimen globally. Recent evidence has shown that dolutegravir, an INSTI which is currently the recommended ART regimens for initial therapy by the WHO guidelines, can be safely co-administered with rifampicin provided they are dose-adjusted to twice daily.22,47,48 The recent Reflate TB2 trial, an open-label, phase 3, randomized clinical trial conducted in Brazil, Côte d’Ivoire, France, Mozambique, and Vietnam comparing another INSTI, raltegravir 400mg BID vs. efavirenz 600mg QD with TDF/3TC in PLHIV on standard TB treatment, failed to demonstrate non-inferiority of raltegravir at week 48.49
The variety and complexity of DDI are summarized in Fig. 3. Such challenges are particularly stark in lower income settings where the range of available ART is limited, rifabutin is frequently not available, HIV viral load monitoring is not universal, and routine genotyping for HIV resistance profiles is generally not available.
When to start TB treatment and ARTIn patients diagnosed with active TB prior to the initiation of ART, the decision of when to start ART is informed by the risk of further immune decline, additive opportunistic infections and death with delaying ART initiation vs. the risk of TB-IRIS, which is greater with earlier ART initiation.50 Several randomized controlled trials have attempted to address the optimal timing of ART initiation. Three large randomized controlled trials, demonstrated that early ART in those with CD4 counts <50cell/μL significantly reduced AIDS events or deaths.50 In patients with CD4 cell counts <50cells/μL therefore, ART initiation is recommended within the first 2 weeks of TB treatment. For patients with CD4 >50cell/μL, ART initiation should be within eight weeks of starting anti-TB therapy.46 These recommendations also apply to patients with suspected or confirmed MDR-TB.46 A randomized placebo-controlled trial conducted in South Africa, Tanzania, Uganda and Zambia in 2014, and randomizing 1675 patients with CD4 counts of 220cells/μl or more to early ART vs ART delayed at the end of six months of TB treatment, did not find differences in TB treatment failure, TB recurrence or death. These results suggest that ART can be delayed until the completion of TB treatment among this subgroup of patients and challenge the current WHO recommendations.51
TB immune reconstitution inflammatory syndromeTB-IRIS is caused by ART-induced restoration of TB-specific immune responses, resulting in either the deterioration of a treated infection (paradoxical IRIS) or a new presentation of a previously subclinical infection (unmasking IRIS). IRIS has been reported in 8–40% following ART initiation.52 Predictors of IRIS include a baseline CD4 count <50cells/μL; rapid on-ART restoration of CD4 counts; high pre-ART and lower on-ART HIV viral loads; and severity of TB disease. Most TB-IRIS occurs within 3 months of the start of ART, usually within the first month. Incidence of unmasking TB-IRIS is harder to quantify but there is concern that the recent rapid upscaling of ‘HIV test and start,’ could result in an increased incidence of unmasking IRIS events to opportunistic infections.53
TB-IRIS ranges from mild to severe to life-threatening. Patients with mild or moderately severe IRIS can be managed symptomatically. For management of severe TB-IRIS, corticosteroids are recommended as demonstrated in a recent RCT, although data on the optimal dose, duration, and overall safety and efficacy are limited.54 In the presence of IRIS, ideally neither TB therapy nor ART should be stopped, as immune restoration and effective control of the bacillary burden are both essential to long term survival.
TB-IRIS prevention with prednisolone prophylaxis (40mg/day for 2 weeks then 20mg/day for 2 weeks started with ART) was investigated in a recent clinical trial among ART-naïve adults at high risk of TB-IRIS (within 30 days of TB treatment initiation and CD4 count ≤100cells/μL). Grade 3 adverse events occurred more frequently in the placebo arm (45.4% vs. 29.4%, p=0.01), but grade 4 adverse events were similar by arm (8.4% vs. 7.6%, p=0.81). The intervention reduced the risk of TB-IRIS by 30% and further reduced the requirement for corticosteroids to treat TB-IRIS by 53%.55
Present areas of uncertaintyTuberculous meningitis is the most severe form of HIV-associated TB. TBM-associated mortality is 40–58% in PLHIV. Given that the risks of TB-IRIS is significantly greater in CNS disease, caution with ART initiation is required. In a study conducted in Vietnam, TBM patients were randomized to immediate ART or to ART deferred 2 months after initiation of TB treatment. A significantly higher rate of severe adverse events was seen in patients who received immediate ART than in those with deferred therapy (80.3% vs. 69.1% for early and deferred ART, respectively; p=0.04).56 There have been no subsequent studies to further delineate the optimum timing of ART in TBM and current expert opinion is to start at 8 weeks. The use of adjuvant steroids in the treatment of extrapulmonary TB disease, namely TBM and pericardial TB, also requires further study in PLHIV. In TBM, the WHO currently recommends initial adjuvant dexamethasone or prednisolone tapered over 6–8 weeks. However, of the nine trials which demonstrated a mortality benefit of adjunctive steroids in the treatment of TBM,57 only one trial from Asia included PLHIV. In the sub-group analysis of PLHIV, there was a non-significant reduction in mortality and no reduction in long term neurological disability.58 Although adjunct steroids remain standard of care for patients with HIV-associated TBM, a currently recruiting study in PLHIV will provide important trial data to further inform clinical practice.59 Finally, an Indian trial randomizing patients with TBM to receive aspirin or placebo as adjuvant to antitubercular treatment showed an absolute risk reduction of stroke in 19% and a 21.7% reduction in three-month mortality.60
There is considerable hope that scale up of HIV “test and start” and IPT uptake will reduce TB/HIV incidence and mortality. However, ongoing evolution of MTB and HIV drug resistance is a competing challenge for which potent, tolerable, affordable and available drugs are the only answer. Moreover, IRIS is likely to pose an ongoing clinical challenge for which other safe host-directed therapies are required.
Challenges of TB/HIV co-infection in resource-limited settingsThe deadly TB/HIV association remains a major public health concern in RLS. Still today, TB is the main cause of morbidity and mortality amongst PLHIV. In turn, HIV represents the main TB epidemic driver in certain regions. TB develops more frequently, recurs more likely and often remains undiagnosed and untreated in PLHIV.1,61 This threat is exacerbated by the growing prevalence of DR-TB. Since 2004, the WHO recommends a’collaborative HIV/TB activities’ package consisting in integrating service delivery, reducing TB burden through early ART initiation and reducing HIV burden in patients with presumptive and diagnosed TB62 (Table 1). In RLS, the clinical challenges managing HIV/TB co-infection are aggravated by programmatic issues related to outdated policies and practices. Improved diagnostics, cheaper, more effective anti-TB regimens and improved ART coverage are some of the main challenges ahead alongside limitations in human, financial resources and health infrastructure.63
EndTB strategy pillars and components.
| Pillars | Components |
|---|---|
| Integrated, patient-centered care and prevention | Early diagnosis of tuberculosis including universal drug-susceptibility testing,and systematic screening of contacts and high-risk groupsTreatment of all people with tuberculosis including drug-resistant tuberculosis, and patient supportCollaborative tuberculosis/HIV activities, and management of co-morbiditiesPreventive treatment of persons at high risk, and vaccination against tuberculosis |
| Bold policies and supportive systems | Political commitment with adequate resources for tuberculosis care and preventionEngagement of communities, civil society organizations, and public and private care providersUniversal health coverage policy, and regulatory frameworks for case notification, vital registration, quality and rational use of medicines, and infection controlSocial protection, poverty alleviation and actions on other determinants of tuberculosis |
| Intensified research and innovation | Discovery, development and rapid uptake of new tools, interventions and strategiesResearch to optimize implementation and impact, and promote innovations |
Adapted from WHO http://www.who.int/tb/post2015_TBstrategy.pdf.
In 2017 in the highest TB/HIV burden countries, 66% of patients with TB were aware of their HIV status.1 However, merely 46% of estimated incident TB cases amongst PLHIV were reported, leaving an enormous gap of PLHIV with non-identified TB, despite systematic TB screening for PLHIV is broadly recommended. Diagnostic gaps remain a big challenge, the cost-effectiveness of Xpert MTB/RIF is controversial, and only half of the countries recommending initial Xpert MTB/RIF testing in PLHIV are implementing it.64–66 With advanced HIV disease rates globally plateauing and being TB a leading cause of death in these patients, the effective implementation of newer tools like TB-LAM, proven to reduce mortality, are essential.64,66 However, despite the WHO recommendation, TB-LAM implementation is rare, not included in guidelines and often confined to research use.67 Also, TB diagnostics in children, access to child-friendly drug formulations, drug-sensitivity testing and access to second line anti-TB drugs pose additional challenges.39,68,69
In addition, despite knowing that ART reduces TB risk, ART coverage among TB-patients remains suboptimal in RLS. The wide and safe implementation of the “Test and Start” strategy together with the roll-out of new ARVs such as Dolutegravir could further impact TB incidence.
Moreover, as mentioned, only a third of countries with TB prevention recommendations are implementing latent TB treatment among PLHIV. Despite shorter effective strategies are available uptake is still limited.70–73
ART and TB service decentralization, task-shifting and one-stop integrated services increases uptake, improves outcomes and lowers patient costs.74–80 Nevertheless, in many settings and in particular in West and Central Africa, with very low ART coverage and centralized and vertical TB and HIV programs, dedicated efforts to HIV/TB integration should be considered.81 Finally, TB screening as part of services like ante-natal care remains challenging.82 Additional barriers relate to funding, being HIV/TB integration the TB programmatic component with lower funding allocation. This leaves countries relying on partnerships with other departments or organizations.1 Furthermore, monitoring and evaluation systems are often not in place; improvements on indicators measuring ART impact and TB prevention are required. Collaborative TB/HIV activities are a pillar of the EndTB strategy yet, outcome data in RLS is lacking83 (Table 1). Finally, social barriers related to health seeking behavior, stigma or lack of community mobilization are not always addressed. Research agendas in RLS should aim to urgently answer these remaining gaps.
ConclusionsMore than twenty years after the advent of ART, and despite wide effective roll-out in resource-limited settings during the past two decades, TB-HIV co-infection remains as a significant global health challenge. TB-HIV co-infection affects millions of people worldwide and threatens global public health. Several barriers exist for the delivery of optimal patient centered TB and HIV prevention and care. Joint action between TB and HIV programs is needed to overcome these barriers and reduce the global burden of this deadly interaction. Fighting TB/HIV co-infection requires strong political commitment to adopt a comprehensive service integration. While countries are making progress, much work remains to be done to ensure policy adoption and implementation across all communities globally.
FundingEL is supported by a Beatriu de Pinós Grant from the Agency for Management of University and Research Grants of the Generalitat de Catalunya (AGAUR, 2016 BP 00204). JMM received a personal 80:20 research grant from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, from 2017 to 2019. FVC is supported by a Wellcome Clinical PhD Fellowship.
Conflict of interestsThe authors declare not having conflicts of interest related to this work. EL has received consulting honoraria from ViiV Healthcare. JMM has received consulting honoraria and/or research grants from AbbVie, Angelini, Bristol-Myers Squibb, Contrafect, Janssen, Genentech, Gilead Sciences, Medtronic, Merck, Novartis, Pfizer, and ViiV Healthcare, outside the submitted work.
ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya.